Importance of Biochemistry Final
- 1. INTRODUCTION to BIOCHEMISTRY Prepared by Dr. Chandrajiit Singh Food Scientist Krishi Vigyan Kendra ŌĆōRewa (MP) E-mail: chandar30@gmail.com
- 2. Introduction to Biochemistry ŌĆó Bio + Chemistry ŌĆó Bio means life ŌĆó Chemistry is about chemicals, their reactions, interactions, principles, process and methods sustaining life.
- 3. Definition of Biochemistry Therefore we can say : ŌĆ£It is a branch of science which deals with study of bio-molecules and their role in living systemŌĆØ or ŌĆ£Chemistry of Life or Living Being is BiochemistryŌĆØ or Biochemistry is a branch of science that seeks to describe the structure, organization and functions of living matter in molecular terms.
- 4. Living Things CanŌĆ” 1. They are complicated and highly organized. 2. Each part of a living organism appears to have a specific purpose of function 3. They are able to extract energy from the environment 4. They are capable of reproducing themselves through generations 5. They exhibit common properties of living matter
- 5. History of Biochemistry ŌĆó Karl Scheele ŌĆō Swedish founder of biochemistry. He studied the chemical composition of matter in mid 1700. ŌĆó Schleiden & Schwann ŌĆō formulated the cell theory in 1840. ŌĆó Walter Flemming ŌĆō discovered chromosomes in 1875 ŌĆó Carl Newberg ŌĆō a German scientist who coined the word biochemistry
- 6. History of Biochemistry ŌĆó Hans Kreb ŌĆō Proposed the Kreb cycle of the TCA in 1937. ŌĆó Embden & Mayerhoff ŌĆō described the glycolytic pathway in 1925. ŌĆó James Watson & Francis Crick ŌĆō described the double helical structure of DNA in 1953
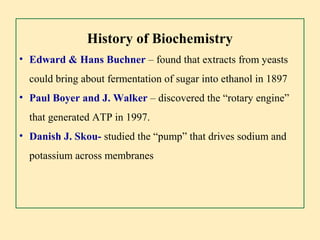
- 7. History of Biochemistry ŌĆó Edward & Hans Buchner ŌĆō found that extracts from yeasts could bring about fermentation of sugar into ethanol in 1897 ŌĆó Paul Boyer and J. Walker ŌĆō discovered the ŌĆ£rotary engineŌĆØ that generated ATP in 1997. ŌĆó Danish J. Skou- studied the ŌĆ£pumpŌĆØ that drives sodium and potassium across membranes
- 8. History of Biochemistry ŌĆó Stanley Prusiner ŌĆō discovered the organism that caused ŌĆ£mad cow disease.ŌĆØ ŌĆó Ruska, et.al. ŌĆō discovered the electron microscope and provided a whole new level of insight into cellular structure.
- 9. Important Dates in Biochemistry
- 10. Important Dates in Biochemistry
- 11. THREE PRINCIPLE AREA OF BIOCHEMISTRY It is divided into 3 principal areas: 1. Structural chemistry 2. Metabolism 3. Chemistry of molecular genetics
- 12. Bio-Molecules A bio-molecule or biological molecule is any molecule that is present in living organisms, including Large Macromolecules Such as proteins, carbohydrate, lipids, and nucleic acids, Small Molecules such as primary metabolites, secondary metabolites, and natural products.
- 13. Metabolites (Small Molecules) Metabolites are small molecules which are the intermediates and products of metabolism Function of Metabolites: Stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, ordants, and pheromones)
- 14. Types of Metabolites 1. Primary Metabolites is directly involved in normal "growth", development, and reproduction. Eg. Ethylene produced in large-scale by Industrial Microbiology. 2. Secondary Metabolites: A secondary metabolite is not directly involved in those processes, but usually has an important ecological function and plant defense against herbivory. Eg. include antibiotics and pigments such as resins and terpenes etc.
- 15. Importance of Macromolecules: 1. Essential structures for the basis of life 2. Control and regulate these processes 3. Responsible for energy exchanges, irritability, metabolism, mobility and reproduction
- 16. Importance of Biochemistry In Agriculture 1. In agriculture biochemistry plays a valuable role in farming, fishery, poultry, sericulture, beekeeping etc. 2. Crop improvement with specific character. 3. Improve in productivity of specific crop. 4. Study the interaction of different pesticide molecules on pests, their behaviour and control. 5. Toxicological effect of pesticide on human and animals for safety purpose.
- 17. Importance of Biochemistry In Agriculture 6. Effect of use and method of use of nutrients and micronutrients (Fertilizer and Bio- fertilizer) on productivity and quality improvement of crop and consumer safety. 7. Effect and changes during storage of agriculture produce and products, eg: ripening of fruits and spoilage. 8. Effect of preservatives and methods employed to enhance shelf life of agricultural produce and products. 9. Effect of nutrients and nutrition on human being.
- 18. Chemical Composition of Living Matter ŌĆó Water ŌĆō 70-90% (free and bound water) ŌĆó Solids ŌĆō 10-30% ŌĆó Inorganic substances ŌĆō 1% (Na, K, Ca, Mg, NH4, Cl- , SO4, PO4 -3 , CO3 -2 , etc. ŌĆó Traces of Fe, I2, Cu, Mn, Co, Zn are also present in combination with organic radicals ŌĆó Rest- organic substances
- 19. Definition of Water Water is a transparent , odorless, tasteless liquid, a compound is a transparent, odorless, tasteless liquid, a compound of hydroge n and oxygen, H2O, freezing at 32┬░F or 0┬░C and boiling at 212┬░F or 100┬░C, in a more or less impure state constitutes rain, oceans, lakes, rivers, etc. It contains 11.188 % Hydrogen and 88.12 % Oxygen by weight.
- 20. Water 1. This is the major component of the cell and is often referred to as an inert space filter in a living organism. 2. It is a strong dipole and has a high dielectric constant. 3. It is highly reactive with unusual properties different physically and chemically from other common liquids. 4. Water and its ionization products H+ and OH- are important factors in determining the structure and biological properties of proteins, nucleic acids, lipids, and other cell components.
- 21. Physical Properties of Water Water has several other unique physical properties. These properties are: 1. Water has a high specific heat. Specific heat is the amount of energy required to change the temperature of a substance. Because water has a high specific heat, it can absorb large amounts of heat energy before it begins to get hot.
- 22. Physical Properties of Water (Continued ŌĆ”.) 2. Water in a pure state has a neutral pH. As a result, pure water is neither acidic nor basic. Water changes its pH when substances are dissolved in it. 3. Water conducts heat more easily than any liquid except mercury. 4. Hydrogen bonds account for the surface tension, viscosity, liquid state at room temperature, and solvent power of water.
- 23. Physical Properties of Water (Cont.) 4. Water molecules exist in liquid form over an important range of temperature from 0 - 100┬░ Celsius. This range allows water molecules to exist as a liquid in most places on our planet. 5. Water is a universal solvent. 6. Water has a high surface tension.
- 24. Chemical Properties of Water 1. WaterŌĆÖs chemical formula is H2O. 2. The water molecule odd shape with both hydrogen atoms occurring on the same side of the oxygen atom gives water its ability to ŌĆ£stickŌĆØ to itself and to other surfaces. 3. The hydrogen atoms create a positive electrical charge while the oxygen atom creates a negative charge. The attraction to one another is what causes water to form droplets.
- 25. Properties of Water of Biological Importance 1. Adhesion: water tends to stick unlike substances . Example is water sticking to blood vessels. 2. Cohesion: which water molecules clings together due to Hydrogen bonding; the surface film (top layer of water) is held by surface tension. Example is spilled water forming a puddle. 3. Solvency: water is considered a universal solvent for its ability to dissolve a wide range of substance since it is a polar
- 26. Properties of Water of Biological Importance (Cont.) 1. It is a universal solvent 2. It is an ideal biologic agent or medium for the ionization of substances and therefore hastens chemical reactions. 3. It has a high specific heat, that is, it takes up more heat to raise its temperature through 1o C, thus allowing the body to store heat effectively without greatly raising its temperature. 4. It possesses a high latent heat of evaporation 5. It has the capacity to conduct heat readily
- 27. Properties of Water of Biological Importance (Cont.) 1. It has a high specific heat, that is, it takes up more heat to raise its temperature through 1o C, thus allowing the body to store heat effectively without greatly raising its temperature. 2. It possesses a high latent heat of evaporation 3. It has the capacity to conduct heat readily.
- 28. Hydrogen Ion Concentration (pH) ŌĆó pH is the negative log of the hydrogen ion concentration. ŌĆó pH = -log(H+ ) ŌĆó Low H values correspond to high concentration of H+ and high pH values correspond to low concentrations of H+ . ŌĆó Acids are proton donors and bases are proton acceptors ŌĆó Strong acids completely dissociate into anions and cations even in strongly acidic solutions.
- 29. Hydrogen Ion Concentration (pH) 1. Strong bases are completely dissociated at high pH. 2. Many biochemicals are weak acids. 3. HCl and H2SO4are strong acids 4. KOH and NaOH are strong bases 5. Ca(OH)2is a weak base
- 30. Buffer Solution 1. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. 2. Its pH changes very little when a small or moderate amount of strong acid or base is added to it and thus it is used to prevent changes in the pH of a solution. 3. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
- 31. Buffer Solution (Cont.) 1. Many life forms thrive only in a relatively small pH range so they utilize a buffer solution to maintain a constant pH. 2. In nature, the bicarbonate buffering system is used to regulate the pH of blood.
- 32. Preparation of Buffer Solution 1. A buffer is made by mixing a large volume of a weak acid or weak base together with its conjugate. 2. A weak acid and its conjugate base can remain in solution without neutralizing each other. 3. The same is true for a weak base and its conjugate acid.
- 36. How Buffer Works 1.HA (Weak Acid ) + AŌĆō (Conjugate Base) A- + HA 2.Only place change takes place and do not go undergo any reaction. 3.Weak Acid and Conjugate Base do not react as water but remains in water as such.
- 37. How Buffer Works 4. However, weak acid and weak acid remains in high concentration in water. 5. They react with strong acid or base if added without changing the pH.
- 38. Strong Base is Added to a Buffer, Acid will Give Its H+ :
- 39. Strong Acid is Added to a Buffer, Weak Base will React with the H+ from the Strong Acid:
- 40. Thank You