Biosimilars and generics
- 2. BIOLOGICS
- 3. • biologics generals • generics drugs differences with Biosimilars • advantages and disadvantages • regulatory pathways • biosimilar • general concepts • example of biologics vs Biosimilar • immunogenicity problem • post translation modification • different outcomes with biologics • price changes
- 4. BIOLOGICS • biological products, or biologics, are medical products. many biologics are made from a variety of natural sources (human, animal or microorganism). like drugs, some biologics are intended to treat diseases and medical conditions. other biologics are used to prevent or diagnose diseases. examples of biological products include:
- 5. BIOLOGICS
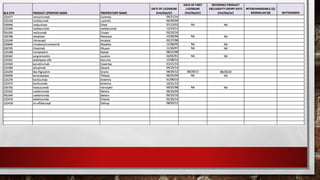
- 7. BIOLOGICS • list of licensed biological products with: • reference product exclusivity • biosimilarity or interchangeability evaluations to date
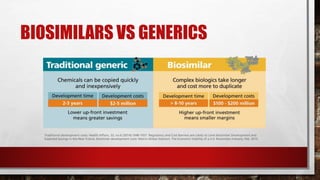
- 12. GENERICS • a generic drug is a medication that has exactly the same active ingredient as the brand-name drug and yields the same therapeutic effect. it is the same in dosing, safety, strength, quality, the way it works, the way it is taken, and the way it should be used. generic drugs do not need to contain the same inactive ingredients as the brand name product. • however, a generic drug can only be marketed after the brand-name drug's patent has expired, which may take up to 20 years after the patent holder’s drug is first filed with the u.s. food and drug administration (fda). •
- 13. GENERICS • generic drugs are usually cheaper than brand name drugs once they reach the market. • a drug company develops new drugs as brand-name drugs under patent protection. this protects their investment in drug research by giving the drug company the sole right to manufacture and sell the brand-name drug while the patent is in effect. • biopharmaceuticals such as monoclonal antibodies they also have generic versions of themselves, known as biosimilars, are typically regulated under an extended set of rules.
- 14. REGULATORY PATHWAYS OF GENERIC DRUGS • generic drug is the same as the brand- name drug (reference listed drug) in terms of the following: • active ingredient; • dosage form (eg, tablet with tablet, not capsule with tablet); • strength (eg, 40 mg referencelisted drug with 40 mg generic); • route of administration; • manufacturedunder the fda’s strict standards of good manufacturing practice regulations;
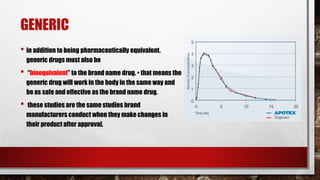
- 15. GENERIC • in addition to being pharmaceutically equivalent, generic drugs must also be • “bioequivalent” to the brand name drug. • that means the generic drug will work in the body in the same way and be as safe and effective as the brand name drug. • these studies are the same studies brand manufacturers conduct when they make changes in their product after approval.
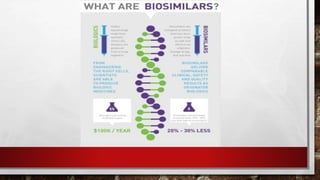
- 16. BIOSIMILAR there are two new typesof biological products- biosimilar and interchangeable. biosimilars are a type of biological product that are licensed (approved) by fda because they are highly similar to an already fda-approved biological product, known as the biological reference product (reference product), and have been shown to have no clinically meaningful differences from the reference product.
- 17. COMPARISON OF BIOSIMILARS AND BIOLOGICS
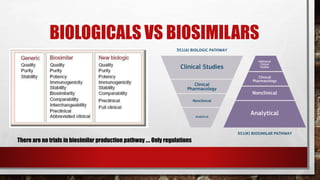
- 19. BIOLOGICALS VS BIOSIMILARS There are no trials in biosimilar production pathway …. Only regulations
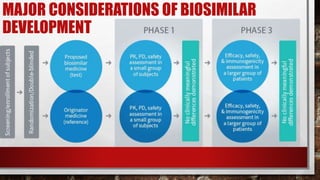
- 21. MAJOR CONSIDERATIONS OF BIOSIMILAR DEVELOPMENT
- 22. THE EMA DEVELOPMENT AND APPROVAL PATHWAY FOR BIOSIMILARS: A STEPWISE APPROACH TO DEMONSTRATION OF BIOSIMILARITY BETWEEN A BIOSIMILAR AND THE ORIGINATOR REFERENCE PRODUCT.
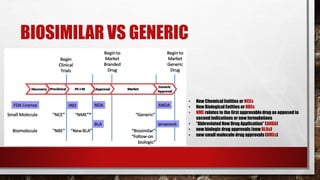
- 23. BIOSIMILAR VS GENERIC • New Chemical Entities or NCEs • New Biological Entities or NBEs • NME relates to the first approvabledrug as opposed to second indications or new formulations • “Abbreviated New Drug Application” (ANDA) • new biologic drug approvals (new BLAs) • new small molecule drug approvals (NMEs)
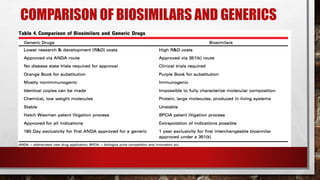
- 24. COMPARISON OF BIOSIMILARS AND GENERICS
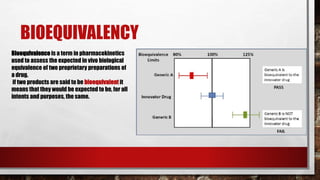
- 27. • two of the most important and most difficult aspects of biosimilar clinical testing are Bioequivalency (purity and potency) and Immunogenicity (safety).
- 28. BIOEQUIVALENCY Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. if two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
- 29. BIOSIMILARITY • Biosimilars are not true generics in the sense that they are not exactly the same as the innovator product, but rather are only considered biologically and clinically comparable to the innovator product.
- 30. IMMUNOGENICITY • immunogenicity is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human or animal. in other words, immunogenicity is the ability to induce a humoral and/or cell-mediated immune responses.
- 32. Differentiation has to be made between wanted and unwanted immunogenicity. • wanted immunogenicity is typically related with vaccines, where the injection of an antigen aiming at protecting the organism. vaccine development is a complex multistep process, immunogenicity being at the center of the vaccine efficiency. • unwanted immunogenicity is an immune response by an organism against a therapeutic antigen (ex. recombinant protein, or monoclonal antibody). this reaction leads to production of anti-drug-antibodies inactivating the therapeutic effects of the treatment and, in rare cases, inducing adverse effects.
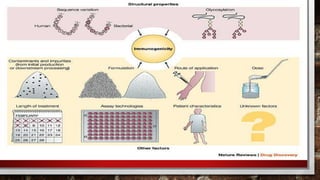
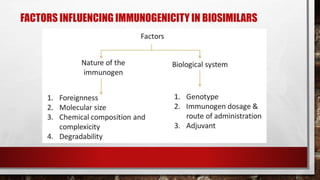
- 33. FACTORS INFLUENCING IMMUNOGENICITY IN BIOSIMILARS
- 34. PATIENT-DISEASE related • genetic factors • disease • activated immune systems due to chronic infections, allergies and autoimmune diseases • age • age-dependent maturation of the immune system observed in children, • potentially altered immune response in the elderly
- 35. PRODUCT RELATED • interactions between proteins and excipients, and impurities from the manufacturing process • packaging may trigger immune responses • Mollecule design • major modification of the therapeutic protein by • glycosylation, • pegylation • formation of fusion proteins
- 36. POST TRANSLATION MODIFICATION • post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins during or after protein biosynthesis. proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. • Biosimilars also go under PTM duo the fact the are mostly proteins which may lead to different outcomes clinically
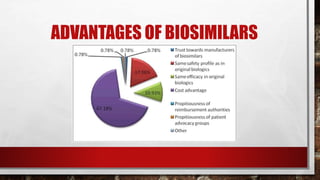
- 37. BIOSIMILAR COMPETITION HAS LED TO CONSISTENT PRICE REDUCTION IN EUROPE • 1. Biosimilars reduced the average list prices for the whole product class in European countries. • 2. lower reference product pricing lowered biosimilar market penetration. • 3. in therapeutic classeswith multiple biosimilar competitors, the first biosimilar to market typically has the highest biosimilar market share • 4. biosimilar competition increased consumption of drug products across several therapeutic.
- 38. CONCLUSION • biosimilars just like generics are a way to a better health care system where almost every human being can benefit from them biologics • cost efficiency can not be denied • more development companies can work and that means future
- 40. QUESTIONS • 1: WHAT ARE BIOSIMILARS? • 2: WHAT'S THE DIFFERENCE BETWEEN BIOSIMILARS AND BIOLOGICS ? • 3: DIFFERENCE BETWEEN GENERICS AND BIOSIMILARS? • 4: WHAT IN POST TRANSLATION MODIFICATION? • 5: WHAT IS WANTED IMMUNOGENICITY?