Full report gas absorption
- 1. UNIVERSITI TEKNOLOGI MARA FAKULTI KEJURUTERAAN KIMIA ENGINEERING CHEMISTRY LABORATORY (CHE485) No. Title Allocated Marks (%) Marks 1 Abstract/Summary 5 2 Introduction 5 3 Aims 5 4 Theory 5 5 Apparatus 5 6 Methodology/Procedure 10 7 Results 10 8 Calculations 10 9 Discussion 20 10 Conclusion 5 11 Recommendations 5 12 Reference / Appendix 5 13 SupervisorŌĆÖs grading 10 TOTAL MARKS 100 Remarks: Checked by : Rechecked by: --------------------------- --------------------------- Date : Date : NAME : SHAZLIYANA BINTI SUZALI STUDENT NO. : 2013830758 GROUP : EH221 4A EXPERIMENT : LAB 1: GAS ABSORPTION COLUMN DATE PERFORMED : 10TH . MARCH ,2015 SEMESTER : 4 PROGRAMME / CODE : EH221 SUBMIT TO : MISS HABSAH ALWI
- 2. 1 TITLE PAGE Abstract 2 Introduction 3 Aims 4 Theory 4 Apparatus and Materials 5-6 Methodology 7-8 Results 9- Calculations 10-13 Discussion 12 Conclusion 15 Recommendations 15 Referemces 16 Appendix 17 ABSTRACT Gas absorption is a process in which the soluble parts of mixture are transferred to a liquid.Gas absorption is done in a packed column .This report are done as to examine the air pressure drop across the column as a function of air flow rate for different water flow rates through the column. When the air pressure drop to certain limit, the phenomena known as ŌĆśfloodingŌĆÖ will occur in which the system can no longer operate as it is. Hence
- 3. 2 the ŌĆśflooding pointŌĆÖ is to be determined as to make sure that the process should be made to operate under the ŌĆśflooding pointŌĆÖ. INTRODUCTION Gas absorption is a process in which the soluble parts of a gas mixture are transferred to or dissolved in a liquid. The reverse process, called desorption or stripping, is used to transfer volatile parts from a liquid mixture to a gas [1] . Therefore there will be mass transfer of the component of the gas from the gas phase to
- 4. 3 the liquid phase. The solute transferred is said to be absorbed by the liquid. In gas desorption (or stripping), the mass transfer is in the opposite direction, of which the transfer is from the liquid phase to the gas phase. The principles for both systems are the same. But from here and on, we are only interested in gas absorption . There are 2 types of absorption processes: physical absorption and chemical absorption,depending on whether there is any chemical reaction between the solute and the solvent(absorbent). When water and hydrocarbon oils are used as absorbents, no significant chemical reactions occur between the absorbent and the solute, and the process is commonly referred to as physical absorption. When aqueous sodium hydroxide (a strong base) is used as the absorbent to dissolve an acid gas, absorption is accompanied by a rapid and irreversible neutralization reaction in the liquid phase and the process is referred to as chemical absorption or reactive absorption. AIMS To examine the air pressure drop across the column as a function of air flow rate for different water flow rates through the column. THEORY Another definition of gas absorption/desorption is ,a process in which a gaseous mixture is brought into contact with a liquid and during this contact a component is transferred
- 5. 4 between the gas stream and the liquid stream. The gas may be bubbled through the liquid, or it may be passed over streams of the liquid, arranged to provide a large surface through which the mass transfer can occur. The liquid film in this latter case can flow down the sides of columns or over packing, or it can cascade from one tray to another with the liquid falling and the gas rising in the counter flow. The gas, or components of it, either dissolves in the liquid (absorption) or extracts a volatile component from the liquid (desorption). [2] In every packed tower with a given size of packing and type , has an upper limit to the rate of gas flow known as flooding velocity of which the tower cannot operate above the velocity mentioned earlier. At low gas velocities the liquid flows downward through the packing uninfluenced by the upward gas flow. As the gas flow rate increases at low gas velocities the pressure drop starts to rise at higher rate. The liquid accumulation increases as the gas flow rate is increased . At the flooding point, the liquid will no longer have the ability to flow down through the pack column and later is blown out with or by the gas. [3] APPARATUS The apparatus used in this experiment are: ’ü¼ SOLTEQ-QVF Absorption column (Model: BP 751-B) ’ü¼ The material used in this experiment is water and air.
- 6. 5
- 7. 6 METHODOLOGY / PROCEDURES A) General start-up 1. All vavlves are closed except the ventilation valve V13. 2. All gas connections are ensured of properly fitted. 3. The valve on the compressed air supply line is opened. The supply pressure is setted up in between 2 to 3 bar by turning the regulator knob clockwise. 4. The shut-off valve on CO2 gas cylinder is opened. The CO2 gas cylinder pressure is ensured to be sufficient. 5. The power for the control panel is turned on. B) Experimental Procedures : Hydrodynamic of a Packed Column (Wet Column
- 8. 7 Presssure Drop) 1. The general start up procedures are carried out 2. The receiving vessel B2 is filled with 50 L of water by opening valve V3 and V5. 3. Valve V3 is closed. 4. Valve V10 and valve V9 are slightly opened. The flow of water from vessel B1 through pump P1 is observed. 5. Pump 1 is switched on then valve V11 is slowly opened and adjusted to give a water flow rate of around 1 L/min. Water is allowed to enter the top of column K1, flow down the column and accumulate at the bottom until it overflows back to vessel B1. 6. Valve 11 is opened and and adjusted to give a water fow rate of 0.5 L/min into column K1. 7. Valve V1 is opened and adjusted to give an air flow rate of 40L/min into column K1. 8. The liquid and gas flow in the column 1 are observed , the pressure drop across the column at dPT-201 is recorded. 9. Steps 6 to 7 are repeated with different values of air flow rate, where each time is increased by 40L/min while the same water flow rate is maintained. 10. Steps 5 to 8 are repeated with different values of water flow rate, of which each time is increased by 0.5L/min by adjusting valve 11. C) General Shut-Down Procedures 1. Pump 1 is switched off. 2. Valves V1,V2 and V3 are closed 3. The valves on the compressed air supply line is closed and the supply pressure is
- 9. 8 exhausted by turning the regulator knob counterclockwise all the way. 4. The shut-off valves on CO2 gas cylinder is closed 5. All liquid in the column in K1 is drained by opening valve V4 and V5. 6. All iquid from receiving vessels B1 and B2 are drained by opening valves V7 and V8. 7. All liquid from pump 1 is drained by opening valve V10. 8. The power for the control panel is turned off. RESULTS AND CALCULATIONS Table 1: Pressure Drop for Wet column Flow rate (L/min) Pressure drop (mmH2O) Air water 20 40 60 80 100 120 140 160 180 1.0 0 2 6 7 9 10 18 21 58 2.0 8 2 0 3 9 30 53 - - 3.0 0 2 7 13 39 - - - -
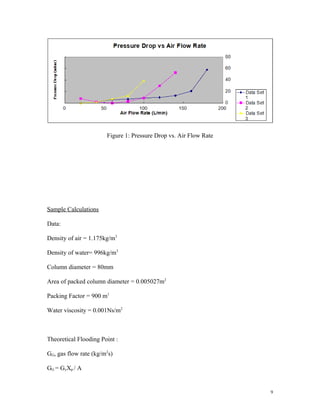
- 10. 9 Figure 1: Pressure Drop vs. Air Flow Rate Sample Calculations Data: Density of air = 1.175kg/m3 Density of water= 996kg/m3 Column diameter = 80mm Area of packed column diameter = 0.005027m2 Packing Factor = 900 m1 Water viscosity = 0.001Ns/m2 Theoretical Flooding Point : GG, gas flow rate (kg/m2 s) GG = GyXp / A
- 11. 10 = 0050207.0 1000 1175.1 sec60 min1 min 20 3 3 L m m kgL ├Ś├Ś├Ś ŌēĪ =0.0779kg/m2 s Capacity parameter, y-axis = 1.0 2 )( )()(1.13 GLG L L pG PP FG ŌłÆ╬Ī ╬Ī ┬Ą = 1.0 2 )175.1996(175.1 ) 996 001.0 (900)0779.0(1.13 ŌłÆ =0.0154 GL , liquid flowrate per unit column cross-sectional area = A XG pL = 005027.0 1000 1175.1 sec60 min1 min 1 3 3 L m m kgL ├Ś├Ś├Ś = 3.896 x 10-3 kg/m2 Flow parameter , x- axis x-axis = )( L G G L G G Žü Žü
- 12. 11 =3.032 Water Flow Rate (L/min) GL (kg/m2 s) 1.0 3.896 x 10-3 2.0 6.6004 3.0 9.9006 Table 2 : Water Flowrate and GL , Liquid Flowrate per Unit Column Cross-sectional Area Air flow rate (L/min) GG (kg/m2 s) Capacity Paramete r (y-axis) Flow parameter (x-axis) 20 0.0779 0.0154 1.0 LPM 2.0LPM 3.0LPM 40 0.1557 0.0614 3.032 2.9102 4.3653 60 0.2336 0.1383 1.4560 1.4560 2.1840 80 0.3115 0.2459 0.9705 0.009705 1.4557 100 0.3893 0.3841 0.7278 0.5823 1.0917 120 0.4672 0.5532 0.005823 0.4854 0.8735 140 0.6229 0.7531 0.7531 0.4160 0.7279 160 0.6229 0.9832 0.9832 0.3639 0.5459
- 13. 12 Table 3 :Air Flowrate ,gas flow rate (kg/m2 s) abrv. GG ,capacity parameter and flow parameter. Figure 2 : Theoretical Pressure Drop Correlation Chart For Random Packings Water Flow Rate Theoretical Experimental Error (%)
- 14. 13 (L/min) Flooding Air Flowrate (L/min) Flooding Air Flowrate (L/min) 1.0 180 160 11.1 2.0 140 120 14.28 3.0 100 80 20 Table 4 : comparison of theoretical and correlation of flooding point DISCUSSION In this experiment, the interest is to examine the air pressure drop across the column as a function of air flow rate for different water flow rates through the column. The experiment based on the flow rate of liquid and gas in the packed. Firstly the water flow rate is kept constant to 1 L/min and the air flow rate is then recorded after a 1 minute interval. Air flow rate is kept rising at constant by 20 L/min by each 5 minutes. All reading of pressure drop are then recorded until the flooding point is reached. The pressure drop for flow rate of air are 0,2,6,7,9,10,13,21,58 mm H20 respectively to 20,40,60,80,100,120,140,160 and 180 L/min of air. The flow rate of water is then adjusted to 2 L/min, the data recorded are 8,2,0,3,9,30,53
- 15. 14 mm H20 respectively to 20,40,60,80,100,120,140,160 L/min of air. It cannot reach 180 L/min of air flow rate as the water will sprayed out from the column due to the high flow rate. Theoretically, the pressure drop will increase as the air flow rate of air is increased, however at the beginning of the experiment , the pump suddenly failed to work as an overflow occurs hence jeoparding the system of which being experimented. As the water flow rate is increased to 3 L/min, the datas are , 0,2,7,13,39 mm H20 respectively to 20,40,60 and 80 L/min of air . Beyond 80 L/min of air , the flooding occurs. The graph of column Pressure Drop vs. Air Flow Rate is plotted and in which the results from the plotted graph shown the higher the gas flow rate , the higher the pressure drop. For correlated value of the pressure drop,calculations has ben made and a graph of capacity parameter against flow rate parameter is plotted. The capacity parameter is indirectly proportional to flow rate parameter . CONCLUSION In conclusion, the air pressure drop across the column increases as the air flow rate increases as well as the water flow rate through the column. From the experiment, the value of experimental pressure drop is higher compared to the correlated values for packed column. For packed column of water flowrate of 1 LPM, the error invovled is the lowest that is 11.1 %, followed by that of water flowrate of 2 LPM which is 14.28 % . At water flowrate of 3 LPM, the error involved is 20% . These percentage errors between theoretical and correlated calculations of flooding point are slightly high due to some unexpected instrumental error as the pump suddenly shut off in the middle of the experiment. Hence, all instruments must be checked before any experiment is conducted to ensure the accuracy of the outcomes.
- 16. 15 RECOMMENDATIONS Some suggestion in improving the safety are to always check and rectify any leak and all operating instructions supplied with the unit must be carefully read and understood before attempting to operate the unit. Next, be extremely careful when handling hazardous, flammable or polluting materials such as CO2. Make sure the system is sufficiently ventilated when working at atmospheric pressure. REFERENCES [1] Perry, Robert H., and Green. Perry's Chemical Engineers' Handbook. New York: McGraw-Hill, Inc. (1984), pp14-6,18-22-2 [2] Retrieved on 12th March,2015 from http://www.nzifst.org.nz/unitoperations/conteqseparation8.htm [3] Geankoplis, C.J. (2003). Transport Processes and Separation Process Principle, 4th Edition. New York: Prentice Hall,pp657-660 [4] Retrieved on 12th March,2015 from
- 18. 17 APPENDIX



![2
the ŌĆśflooding pointŌĆÖ is to be determined as to make sure that the process should be made
to operate under the ŌĆśflooding pointŌĆÖ.
INTRODUCTION
Gas absorption is a process in which the soluble parts of a gas mixture are transferred to
or dissolved in a liquid. The reverse process, called desorption or stripping, is used to
transfer volatile parts from a liquid mixture to a gas [1]
. Therefore there will be mass transfer of the component of the gas from the gas phase to](https://image.slidesharecdn.com/fullreportgas-absorption-150620121044-lva1-app6891/85/Full-report-gas-absorption-3-320.jpg)

![4
between the gas stream and the liquid stream. The gas may be bubbled through the liquid,
or it may be passed over streams of the liquid, arranged to provide a large surface through
which the mass transfer can occur. The liquid film in this latter case can flow down the
sides of columns or over packing, or it can cascade from one tray to another with the
liquid falling and the gas rising in the counter flow. The gas, or components of it, either
dissolves in the liquid (absorption) or extracts a volatile component from the liquid
(desorption). [2]
In every packed tower with a given size of packing and type , has an upper limit to the
rate of gas flow known as flooding velocity of which the tower cannot operate above the
velocity mentioned earlier. At low gas velocities the liquid flows downward through the
packing uninfluenced by the upward gas flow. As the gas flow rate increases at low gas
velocities the pressure drop starts to rise at higher rate. The liquid accumulation increases
as the gas flow rate is increased . At the flooding point, the liquid will no longer have the
ability to flow down through the pack column and later is blown out with or by the gas.
[3]
APPARATUS
The apparatus used in this experiment are:
’ü¼ SOLTEQ-QVF Absorption column (Model: BP 751-B)
’ü¼ The material used in this experiment is water and air.](https://image.slidesharecdn.com/fullreportgas-absorption-150620121044-lva1-app6891/85/Full-report-gas-absorption-5-320.jpg)









![15
RECOMMENDATIONS
Some suggestion in improving the safety are to always check and rectify any leak and all
operating instructions supplied with the unit must be carefully read and understood before
attempting to operate the unit. Next, be extremely careful when handling hazardous,
flammable or polluting materials such as CO2. Make sure the system is sufficiently
ventilated when working at atmospheric pressure.
REFERENCES
[1] Perry, Robert H., and Green. Perry's Chemical Engineers' Handbook. New York:
McGraw-Hill, Inc. (1984), pp14-6,18-22-2
[2] Retrieved on 12th
March,2015 from
http://www.nzifst.org.nz/unitoperations/conteqseparation8.htm
[3] Geankoplis, C.J. (2003). Transport Processes and Separation Process Principle, 4th
Edition. New York: Prentice Hall,pp657-660
[4] Retrieved on 12th
March,2015 from](https://image.slidesharecdn.com/fullreportgas-absorption-150620121044-lva1-app6891/85/Full-report-gas-absorption-16-320.jpg)

