Hibridisasi
- 1. Topic 13: ORBITAL HYBRIDIZATION: The question of shape! We need next to examine the relationship between: âĒ isolated atoms (with valence eâs in s,p, and d orbitals of specific shapes, see next slide as review!) âĒ bonded atoms in molecules or ions, in which bonded regions exhibit significantly different shapes as described by VSEPR theory Kotz & Treichel, Chapter 10 (10.1-2)
- 2. Orbital shapes, Individual (âisolatedâ) Atoms all s orbitals all p orbitals d orbitals Compare (next slide) to molecule, ion bonding shapes
- 3. trigonal tetrahedral trigonal-bipyramidal octahedral
- 4. To rationalize how the shapes of atomic orbitals are transformed into the orbitals occupied in covalently bonded species, we need the help of two bonding theories: Valence Bond (VB) Theory, the theory we will explore, describes the placement of electrons into bonding orbitals located around the individual atoms from which they originated. Molecular Orbital (MO) Theory places all electrons from atoms involved into molecular orbitals spread out over the entire species. This theory works well for excited species, and molecules like O2. You will meet this theory in advanced classes!
- 5. COVALENT BOND FORMATION (VB THEORY) In order for a covalent bond to form between two atoms, overlap must occur between the orbitals containing the valence electrons. The best overlap occurs when two orbitals are allowed to meet âhead onâ in a straight line. When this occurs, the atomic orbitals merge to form a single bonding orbital and a âsingle bondâ is formed, called a sigma (s) bond.
- 6. Dotted areas: representation of "electron cloud" for one electron "Head-on Overlap" Sigma Bond: merged orbital, 2 e's
- 7. MAXIMIZING BOND FORMATION In order for âbest overlapâ to occur, valence electrons need to be re-oriented and electron clouds reshaped to allow optimum contact. To form as many bonds as possible from the available valence electrons, sometimes separation of electron pairs must also occur. We describe the transformation process as âorbital hybridizationâ and we focus on the central atom in the species...
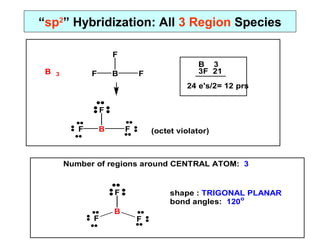
- 8. âspâ Hybridization: all 2 Region Species eB lC 2 lC eB lC Be 2 2Cl 14 16 e's/2= 8 prs lC eB lC (octet violator) Number of regions around CENTRAL ATOM: 2 lC eB lC shape : LINEAR bond angles: 180o
- 9. Hybridization of Be in BeCl2 Atomic Be: 1s2 2s2 Valence eâs Energy 2p 2s separate 2p 2s Hybrid sp orbitals: 1 part s, 1 part p "hybridize" "sp" "sp"
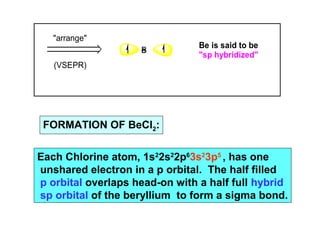
- 10. "arrange" (VSEPR) Be Be is said to be "sp hybridized" FORMATION OF BeCl2: Each Chlorine atom, 1s22s22p63s23p5 , has one unshared electron in a p orbital. The half filled p orbital overlaps head-on with a half full hybrid sp orbital of the beryllium to form a sigma bond.
- 11. lC eB lC lC eB lC lC eB lC s p hybridized, linear, 180o bond angle s
- 12. âsp2â Hybridization: All 3 Region Species FB 3 F B F B 3 3F 21 24 e's/2= 12 prs F B F (octet violator) Number of regions around CENTRAL ATOM: 3 shape : TRIGONAL PLANAR bond angles: 120o F F F B F F
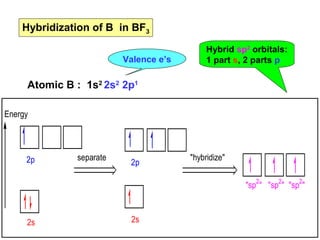
- 13. Hybridization of B in BF3 Valence eâs Atomic B : 1s2 2s2 2p1 Energy 2p 2s separate 2p 2s Hybrid sp2 orbitals: 1 part s, 2 parts p "hybridize" "sp2" "sp2" "sp2"
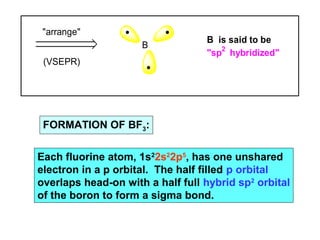
- 14. "arrange" (VSEPR) B B is said to be FORMATION OF BF3: "sp2 hybridized" Each fluorine atom, 1s22s22p5, has one unshared electron in a p orbital. The half filled p orbital overlaps head-on with a half full hybrid sp2 orbital of the boron to form a sigma bond.
- 15. sp2 hybridized, TRIGONAL PLANAR, 120o bond angles F B F F F B F F
- 16. âsp3â Hybridization: All 4 Region Species CH 4 H C H C 4 4H 4 Number of regions around CENTRAL ATOM: 4 shape : TETRAHEDRAL bond angles: 109.5o H H 8 8 e's /2 = 4 pr H C H H H
- 17. Hybridization of C in CH4 Valence eâs Atomic C : 1s2 2s2 2p2 Energy 2p 2s separate 2p 2s Hybrid sp3 orbitals: 1 part s, 3 parts p "hybridize" "sp3" "sp3" "sp3" "sp3"
- 18. "arrange" (VSEPR) FORMATION OF CH4: C C is said to be "sp3 hybridized" Each hydrogen atom, 1s1, has one unshared electron in an s orbital. The half filled s orbital overlaps head-on with a half full hybrid sp3 orbital of the carbon to form a sigma bond.
- 19. sp3hybridized, TETRAHEDRAL, 109.5o bond angles H C H H H H C H H H
- 20. Unshared Pairs, Double or Triple Bonds Unshared pairs occupy a hybridized orbital the same as bonded pairs: See the example of NH3 that follows. Double and triple bonds are formed from electrons left behind and unused in p orbitals. Since all multiple bonds are formed on top of sigma bonds, the hybridization of the single (s) bonds determine the hybridization and shape of the molecule...
- 21. HN 3 H N H N 5 3H 3 8e's/2=4 prs H Number of regions around CENTRAL ATOM: 4 shape : TETRAHEDRAL bond angles: < 109.5o N H H H N H H H
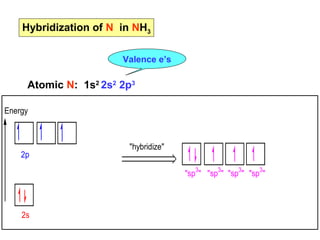
- 22. Hybridization of N in NH3 Valence eâs Atomic N: 1s2 2s2 2p3 Energy 2p 2s "hybridize" "sp3" "sp3" "sp3" "sp3"
- 23. "arrange" (VSEPR) FORMATION OF NH3: N N is said to be "sp3 hybridized" Each hydrogen atom, 1s1, has one unshared electron in an s orbital. The half filled s orbital overlaps head-on with a half full hybrid sp3 orbital of the nitrogen to form a sigma bond.
- 24. sp3hybridized, TETRAHEDRAL, ~107o bond angles N H H H N H H H
- 25. Group Work 13.1 Describe Hybridization of C and shape of following species: CO, CO2, HCN, CH2O, CO3 2- , CBr4 C O O C O H C N O C H H O C O O 2 Br C Br Br Br
- 26. âsp3dâ Hybridization: All 5 Region Species PF 5 F P F P 5 5F 35 F Number of regions around CENTRAL ATOM: 5 shape : TRIGONAL BIPYRAMIDAL bond angles: 90, 120, 180o F F 40 40 e 's /2 = 20 pr P F F P F F F F F F F F
- 27. Hybridization of P in PF5 P: 1s2 2s2 2p6 3s2 3p3 Energy 3d 3d 3p 3s separate 3p 3s "hybridize" "sp3d" "sp3d" "sp3d" "sp3d" "sp3d"
- 28. "arrange" (VSEPR) P is said to be "sp3d P hybridized" FORMATION OF PF5: Each fluorine atom, 1s22s22p5, has one unshared electron in a p orbital. The half filled p orbital overlaps head-on with a half full hybrid sp3d orbital of the phosphorus to form a sigma bond.
- 29. sp3d hybridized, TRIGONAL BIPYRAMIDAL, 90, 120, 180o bond angles F P F F F F F P F F F F
- 30. âsp3d2â Hybridization: All 6 Region Species SF 6 F S F S 6 6F 42 F F F F Number of regions around CENTRAL ATOM: 6 shape : OCTAHEDRAL bond angles: 90, 180o F F 48 48 e 's /2 = 24 pr S F S F F F F F F F F F
- 31. Hybridization of S in SF6 S: 1s2 2s2 2p6 3s2 3p4 Energy 3d 3d 3p 3s separate 3p 3s "hybridize" "sp3d2"
- 32. "arrange" (VSEPR) S is said to be "sp3d2 S hybridized" FORMATION OF SF6: Each fluorine atom, 1s22s22p5, has one unshared electron in a p orbital. The half filled p orbital overlaps head-on with a half full hybrid sp3d2 orbital of the phosphorus to form a sigma bond.
- 33. sp3d hybridized, TRIGONAL BIPYRAMIDAL, 90, 120, 180o bond angles F S F F F F F F F F S F F F
- 34. Group Work 13.2 Describe hybridization of S and shape of species in SF2, SO2, SO3 2- , SF3 +, SF4, SF5 - S F F S O O S O O O S F F F 2- S F F F F F S F F F F
- 35. Summary: Regions, Shapes and Hybridization #, regions shape hybridization 2 linear sp 3 trigonal planar sp2 4 tetrahedral sp3 5 trigonal bipyramidal sp3d 6 octahedral sp3d2
- 36. âBOTTOM LINEâ IF you can draw a Lewis structure for a species, and count electronic regions around central atom, you can immediately determine: âĒ the shape of the species about the central atom âĒ the hybridization of the species based on the central atom See excellent chart, p. 450, Kotz