The Road to Better Traceability
ŌĆó
2 likesŌĆó539 views
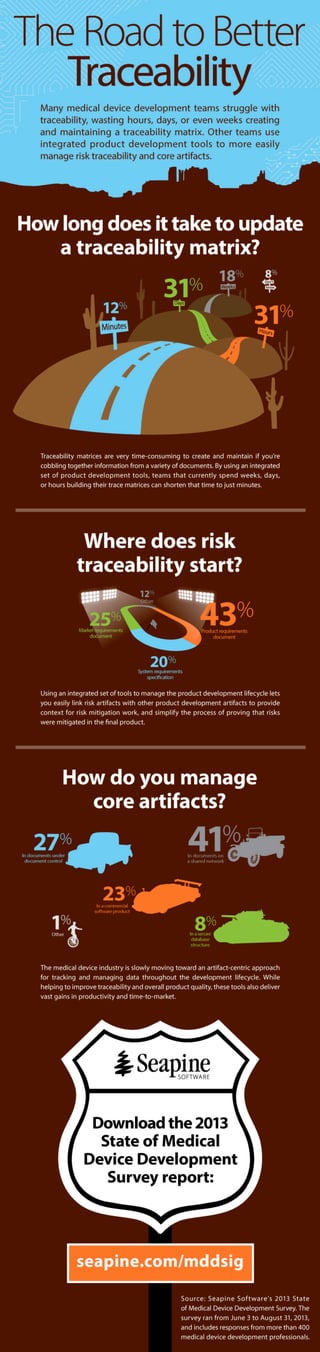
Many medical device development teams struggle with traceability, wasting hours, days, or even weeks creating and maintaining a traceability matrix. Other teams use integrated product development tools to more easily manage risk traceability and core artifacts.
1 of 1
Download to read offline