Defining Human Research
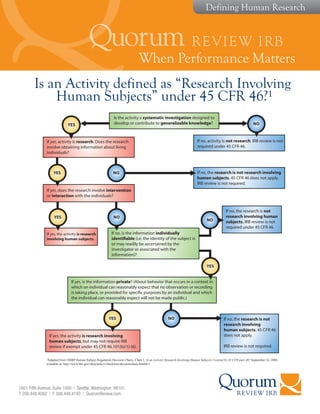
- 1. When Performance Matters 1601 Fifth Avenue, Suite 1000 | Seattle, Washington 98101 T 206.448.4082 | F 206.448.4193 | QuorumReview.com Defining Human Research Is an Activity defined as ŌĆ£Research Involving Human SubjectsŌĆØ under 45 CFR 46?┬╣ Is the activity a systematic investigation designed to develop or contribute to generalizable knowledge? If no, activity is not research. IRB review is not required under 45 CFR 46. If yes, activity is research. Does the research involve obtaining information about living individuals? YES If yes, does the research involve intervention or interaction with the individuals? YES NO If no, the research is not research involving human subjects. 45 CFR 46 does not apply. IRB review is not required. NO If yes, the activity is research involving human subjects. YES YES NO If no, is the information individually identifiable (i.e. the identity of the subject is or may readily be ascertained by the investigator or associated with the information)? NO If no, the research is not research involving human subjects. IRB review is not required under 45 CFR 46. If yes, is the information private? (About behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, or provided for specific purposes by an individual and which the individual can reasonably expect will not be made public.) NO If yes, the activity is research involving human subjects, but may not require IRB review if exempt under 45 CFR 46.101(b)(1)-(6). If no, the research is not research involving human subjects. 45 CFR 46 does not apply. IRB review is not required. YES ┬╣Adapted from OHRP Human Subject Regulation Decision Charts, Chart 1, Is an Activity Research Involving Human Subjects Covered by 45 CFR part 46? September 24, 2004, available at: http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html#c1.