mcq (1).pptx
- 1. ’üČ An electron of mass ŌĆśmŌĆÖ is confined to a one dimensional box of length ŌĆśbŌĆÖ. If it makes a radioactive transition from second excited state to the ground state, the frequency of the photon emitted is- (a) 9h2/ mb2 (b) 3h2/ 8mb2 (c) h/ mb2 (d) 2h/ 8mb2 ANS: (c) ’üČ The function eax2 (a > 0) is not an acceptable wave function for bound system because ŌĆō (a) It is not continuous (b) it is multi- valued (c) it is not normalizable (d) all of these ANS: (d) ’üČ Among the following, the isoelectronic and isostructural pair is- (a) CO2 & SO2 (b) SO3 & SeO2 (c) NO2 + & TeO2 (d) SiO4 4- & PO4 3- ANS: (d)
- 2. ’üČ The HOMO of HF is- (a) bonding (b) antibonding (c) ionic (d) non- bonding ANS: (a) ’üČ The bond angle of Cl2O is- (a) smaller than F2O (b) greater than H2O (c) Smaller than H2O (d) same as that of H2O ANS: (b) ’üČ The species which has a square planar structure is- (a) BF4 - (b) FeCl4 ŌĆō (c) SF4 (d) XeF4 ANS: (d)
- 3. ’üČ Icosahedral structure is generally exhibited by- (a) C (b) Si (c) Ge (d) B ANS: (d) [XeO6]-4 is octahedral whereas XeF6 is distorted one, because- (a) F is more electronegative than O (b) Xe has a lone pair in XeF6 (c) XeF6 is neutral but [XeO6]-4 is anionic (d) Xe-F bond has more ionic characters ANS: (b) ’üČ The ground state term symbol for high spin d5s1 & d5 configuration respectively are- (a) S3 & S6 (b) P6 & S3 (c) S7 & S6 (d) P7 & S6 ANS: (c)
- 4. ’üČ The Xenon compounds that are isostructural with IBr2 - & BrO3 - respectively are ŌĆō (a) linear XeF2 & pyramidal XeO3 (b) bent XeF2 & pyramidal XeO3 (c) bent XeF2 & planar XeO3 (d) linear XeF2 & tetrahedral XeO3 ANS: (a) ’üČ The reaction of solid XeF2 with AsF5 in 1:1 ratio affords- (a) XeF4 & AsF3 (b) XeF6 & AsF3 (c) [XeF]+ [AsF6]- (d) [Xe2F3]+ [AsF6]- ANS: (c) ’üČ White phosphorous P4 belongs to the- (a) Closo- borane (b) nido system (c) arachno system (d) hypo system ANS: (b)
- 5. ’üČ Jahn- Teller distortion of CuSO4 . 5H2O acts to- (a)raise the symmetry (b) remove electronic degeneracy (c) Cause loss of H2O ligand (d) promote a 'dŌĆś electron to an antibonding molecular orbital ANS: (b) ’üČ The complex that exists as a pair of enantiomer is- (a) trans-[Co(H2NCH2CH2NH2)2Cl2]+ (b)cis- [Co(NH3)4Cl2]+ (c) [Pt(PPh3)(Cl)(Br)(CH3)]- (d) [Co(H2NCH2CH2NH2)3]+3 ANS: (d) ’üČ The correct statement about the Cu-N bond distances in [Cu(NH3)6]+2 is- (a) All the bond distances are equal (b) the axial bonddistances are longer than the equatorial ones (c) the equatorial bond distances are longer than the axial ones ANS: (b)
- 6. ’üČ The existance of two different coloured complexes of Co(NH3)4Cl2 is due to- (a) Optical isomerism (b) linkage isomerism (c) geometrical isomerism (d) coordination isomerism ANS: (c) ’üČ The compound which exhibits Jahn- Teller distortion- (a) [Mn(H2O)6]+2 (b) [Mn(H2O)6]+3 (c) [Cr(H2O)6]+3 (d) [Fe(CN)6]-4 ANS: (b) ’üČ The compound which shows LŌåÉM charge transfer is- (a) Ni(CO)4 (b) K2Cr2O7 (c) HgO (d) [Ni(H2O)6]+2 ANS: (a)
- 7. ’üČ The number of absorption bands observed in [FeF6]-3 & [CoF6]-3 respectively are- (a) 1 & 3 (b) 0 & 1 (c) 0 & 3 (d) 3 & 1 ANS: (b) ’üČ An electron is found in an orbital with one radial node and two angular nodes. Which orbital the electron is in- (a) 1s (b) 2p (c) 3d (d) 4d ANS: (d) ’üČ Which one of the following has the highest lattice energy- (a) LiCl (b) CaCl2 (c) LiF (d) KCl ANS: (b)
- 8. ’üČ The fluoride, whose value of dipole moment is not equal to zero is- (a) XeF4 (b) CF4 (c) SF4 (d) PF5 ANS: (c) ’üČ The compound having the highest m.p is- (a) LiCl (b) LiF (c) LiI (d) LiBr ANS: (b) ’üČ The shape of SF4 is- (a) td (b) tbp (c) sq. Planar (d) Oh ANS: (a)
- 9. ’üČ The decreasing order of the (I.E)1 of the following element is- (a) Xe> Be> As> Al (b) Xe> As> Al> Be (c) Xe> As> Be> Al (d) Xe> Be> Al> As ANS: (c) ’üČ The species having angular shape is- (a) BeF2 (b) NO2 - (C) CH2O (d) XeF4 ANS: (b) ’üČ The number of microstates for p2 configuration is- (a) 45 (b) 15 (c) 25 (d) 10 ANS: (b)
- 10. ’üČ BrO3 - is isostructural with a noble gas species- (a) XeO3 (b) XeF3 (c) XeF4 (d) XeO2 ANS: (a) ’üČ The correct order of bond angle in BF3, NH3, NF3 & PH3 are- (a) BF3 > NH3 > NF3 > PH3 (b) PH3 > BF3 > NF3 > NH3 (c) BF3 > PH3 > NH3 > NF3 (d) NH3 > NF3 > BF3 > PH3 ANS: (a) ’üČ In which of the following pairs the species have similar geometry- (a) CO2 & SO2 (b) CO3 - & SO3 -2 (c) NH3 & BH3 (d) SO4 -2 & ClO4 - ANS: (d)
- 11. ’üČ Among Ar, NH4Cl, HF & HCl, the strength of inter atomic or intermolecular forces follow the order- (a) NH4Cl> HF> HCl> Ar (b) HF> HCl> Ar> NH4Cl (c) HCl> Ar> NH4Cl> HF (d) Ar> NH4Cl> HF> HCl ANS: (a) ’üČ The most polar compound amoung the following is- (a) SF4 (b) BF3 (c) XeF4 (d) SO3 ANS: (a) ’üČ The most probable oxidation states of both Cr & Mo are- (a) +2, +3, +4 (b) +2, +3, +5 (c) +2, +3,+6 (d) +3, +4, +5 ANS: (c)
- 12. ’üČ The correct order of acidic character is- (a) Al2O3 > MgO > SiO2 > P4O10 (b) P4O10 > Al2O3 > MgO > SiO2 (c) P4O10 > SiO2 > Al2O3 > MgO (d) SiO2 > P4O10 > Al2O3 > MgO ANS: (c) ’üČ If the values of Madelung constant of the following compounds are equal, then their lattice energy value decreases in order- (a) KCl > NaF > CaO> Al2O3 (b) Al2O3 > CaO > NaF > KCl (c) NaF > KCl > CaO > Al2O3 (d) Al2O3 > Cao > KCl > NaF ANS: (b) ’üČ The correct order of the mean bond energies in following binary hydrides are- (a) CH4 > NH3 > H2O >HF (b) NH3 > CH4 > H2O > HF (c) HF > H2O > CH4 > NH3 (d) HF > H2O > NH3 > CH4 ANS: (d)
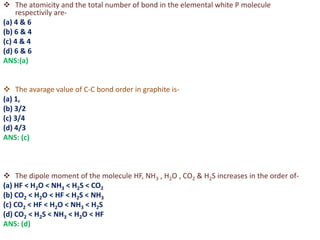
- 13. ’üČ The atomicity and the total number of bond in the elemental white P molecule respectivily are- (a) 4 & 6 (b) 6 & 4 (c) 4 & 4 (d) 6 & 6 ANS:(a) ’üČ The avarage value of C-C bond order in graphite is- (a) 1, (b) 3/2 (c) 3/4 (d) 4/3 ANS: (c) ’üČ The dipole moment of the molecule HF, NH3 , H2O , CO2 & H2S increases in the order of- (a) HF < H2O < NH3 < H2S < CO2 (b) CO2 < H2O < HF < H2S < NH3 (c) CO2 < HF < H2O < NH3 < H2S (d) CO2 < H2S < NH3 < H2O < HF ANS: (d)
- 14. ’üČ About the activity of alkaline earth metal as reducing agent, which of the following statements are true? (a) It decreases from Be to Ba (b) it increases from Be to Ba (c) it increases from Be to Ca & decreases from Ca to Ba (d) it decreases from Be to Ca & increases from Ca to Ba ANS: (c) ’üČ Among the compounds A-D, those which hydrolyse easily are-(A) NCl3 (B) NF3 (C) BiCl3 (D) PCl3 (a) A & B only (b) A, C & D (c) B, C & D (D) A, B & C ANS: (b) ’üČ Among the following, an example of a hypervalent species is- (a) BF3. OEt2 (b) SF4 (c) PF6 - (d) Sb2S3 ANS: (c)
- 15. ’üČ The pair of solvent in which PCl5 does not ionize is- (a) CH3CN, CH3NO2 (b) CH3CN, CCl4 (c) C6H6 , CCl4 (d) CH3CN, C6H6 ANS:(c) ’üČ Among the halides NCl3 (A), PCl3 (B), AsCl3 (C) , those which produce two different acids upon hydrolysis are- (a) A & B (b) A & C (c) B & C (d) A, B & C ANS:(c) ’üČ Triphosphazene is prepared by reacting X & Y in equimolar ratio at 120 ╠Ŗ- 150 ╠Ŗ using appropriate solvents- (a) PCl3 & NH3 (b) PCl5 & NH3 (c) PCl5 & NH4Cl (d) PCl3 & NH4Cl ANS:(c)
- 16. ’üČ Pyroxenes are class of silicate minerales which exhibit a polymeric chain structure, its simplest repeating unit is- (a) [SiO4]-4 (b) [SiO3]-2 (c) [Si2O7]-6 (d) [Si4O11]-6 ANS: (b) ’üČ Which of the following does not exhibit paramagnetism- (a) NO (b) NO2 (c) ClO2 (d) ClO2 - ANS: (b) ’üČ In which of the following, the hybridisation of central atom is other than sp3d (a) PF5 (b) SF4 (c) IF5 (d) I3 + ANS: (c)
- 17. ’üČ Which compound will produce N2 on thermal decomposition- (a) NH4NO2 (b) (NH4)2Cr2O7 (c) NaN3 (d) All of these ANS: (d) ’üČ The bond angle of NF3 (102.3 ╠Ŗ ) is smaller than NH3 (107.2 ╠Ŗ ). This is because- (a) Large size of F compared to H (b) Large size of N compared to H (c) Opposite polarity of N in the two molecules (d) Smaller size of H compared to N ANS: (c)


![’üČ Icosahedral structure is generally exhibited by-
(a) C
(b) Si
(c) Ge
(d) B
ANS: (d)
[XeO6]-4 is octahedral whereas XeF6 is distorted one, because-
(a) F is more electronegative than O
(b) Xe has a lone pair in XeF6
(c) XeF6 is neutral but [XeO6]-4 is anionic
(d) Xe-F bond has more ionic characters
ANS: (b)
’üČ The ground state term symbol for high spin d5s1 & d5 configuration respectively are-
(a) S3 & S6
(b) P6 & S3
(c) S7 & S6
(d) P7 & S6
ANS: (c)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-3-320.jpg)
![’üČ The Xenon compounds that are isostructural with IBr2
- & BrO3
- respectively are ŌĆō
(a) linear XeF2 & pyramidal XeO3
(b) bent XeF2 & pyramidal XeO3
(c) bent XeF2 & planar XeO3
(d) linear XeF2 & tetrahedral XeO3
ANS: (a)
’üČ The reaction of solid XeF2 with AsF5 in 1:1 ratio affords-
(a) XeF4 & AsF3
(b) XeF6 & AsF3
(c) [XeF]+ [AsF6]-
(d) [Xe2F3]+ [AsF6]-
ANS: (c)
’üČ White phosphorous P4 belongs to the-
(a) Closo- borane
(b) nido system
(c) arachno system
(d) hypo system
ANS: (b)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-4-320.jpg)
![’üČ Jahn- Teller distortion of CuSO4 . 5H2O acts to-
(a)raise the symmetry
(b) remove electronic degeneracy
(c) Cause loss of H2O ligand
(d) promote a 'dŌĆś electron to an antibonding molecular orbital
ANS: (b)
’üČ The complex that exists as a pair of enantiomer is-
(a) trans-[Co(H2NCH2CH2NH2)2Cl2]+
(b)cis- [Co(NH3)4Cl2]+
(c) [Pt(PPh3)(Cl)(Br)(CH3)]-
(d) [Co(H2NCH2CH2NH2)3]+3
ANS: (d)
’üČ The correct statement about the Cu-N bond distances in [Cu(NH3)6]+2 is-
(a) All the bond distances are equal
(b) the axial bonddistances are longer than the equatorial ones
(c) the equatorial bond distances are longer than the axial ones
ANS: (b)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-5-320.jpg)
![’üČ The existance of two different coloured complexes of Co(NH3)4Cl2 is due to-
(a) Optical isomerism
(b) linkage isomerism
(c) geometrical isomerism
(d) coordination isomerism
ANS: (c)
’üČ The compound which exhibits Jahn- Teller distortion-
(a) [Mn(H2O)6]+2
(b) [Mn(H2O)6]+3
(c) [Cr(H2O)6]+3
(d) [Fe(CN)6]-4
ANS: (b)
’üČ The compound which shows LŌåÉM charge transfer is-
(a) Ni(CO)4
(b) K2Cr2O7
(c) HgO
(d) [Ni(H2O)6]+2
ANS: (a)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-6-320.jpg)
![’üČ The number of absorption bands observed in [FeF6]-3 & [CoF6]-3 respectively are-
(a) 1 & 3
(b) 0 & 1
(c) 0 & 3
(d) 3 & 1
ANS: (b)
’üČ An electron is found in an orbital with one radial node and two angular nodes. Which
orbital the electron is in-
(a) 1s
(b) 2p
(c) 3d
(d) 4d
ANS: (d)
’üČ Which one of the following has the highest lattice energy-
(a) LiCl
(b) CaCl2
(c) LiF
(d) KCl
ANS: (b)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-7-320.jpg)







![’üČ Pyroxenes are class of silicate minerales which exhibit a polymeric chain structure, its
simplest repeating unit is-
(a) [SiO4]-4
(b) [SiO3]-2
(c) [Si2O7]-6
(d) [Si4O11]-6
ANS: (b)
’üČ Which of the following does not exhibit paramagnetism-
(a) NO
(b) NO2
(c) ClO2
(d) ClO2
-
ANS: (b)
’üČ In which of the following, the hybridisation of central atom is other than sp3d
(a) PF5
(b) SF4
(c) IF5
(d) I3
+
ANS: (c)](https://image.slidesharecdn.com/mcq1-230622151227-cdc6da4f/85/mcq-1-pptx-16-320.jpg)
