Data Quality Pyramid
- 1. GLP Research and Data Quality Under several statutes, government requires Good Laboratory Practice (GLP) studies. Research must follow specified protocols with each step documented. Only GLP qualified facilities and personnel can be used. GLP research is demonstratively valid. In other words, if anyone wishes to conduct the research – then the results should be reproducible. An unintentional GLP violation can invalidate the study. An intentional GLP violation can be a criminal offense. If studies that make the headlines in the news media today were of GLP quality, quite likely the debate we are witnessing would not be occurring.
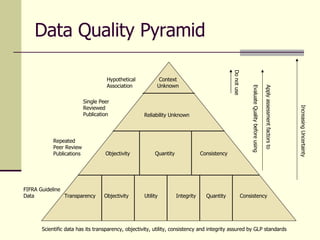
- 2. Data Quality Pyramid Utility Objectivity Transparency Integrity Quantity Consistency Objectivity Quantity Consistency Reliability Unknown Context Unknown Hypothetical Association Single Peer Reviewed Publication Repeated Peer Review Publications FIFRA Guideline Data Do not use Apply assessment factors to Evaluate Quality before using Increasing Uncertainty Scientific data has its transparency, objectivity, utility, consistency and integrity assured by GLP standards
- 3. Data Quality Pyramid for Risk Assessment Processes and Decisions FIFRA Guideline Data: Has its utility defined by FIFRA testing guidelines; has its consistency defined by EPA review; has its quantity defined by FIFRA data requirements; has its objectivity, transparency and integrity assured by GLP requirements. Repeated peer-reviewed publications: Has consistency defined by replication, demonstrates quantity based on the statistical design of the studies; has its objectivity from peer review; has utility to the extent it supports risk assessment; but loses its integrity and transparency because methods are not documented to the degree GLP requires. Single peer-reviewed publications: Has limited objectivity depending on the level of peer review but has its utility defined by one circumstance and may not have been designed for purposes of risk assessment; loses its transparency because methods are not documented to the degree GLP requires; loses its quantity by its isolation and is of unknown consistency . Hypothetical association: Has virtually no utility ; loses its objectivity to subjective speculation; has no transparency in methodological scientific application; is not supported by any quantity of data; and has no measure for consistency .