Energy Unit Basics
- 1. Lonny Grafman, Environmental Resources Engineering
- 2. Basic Units
- 3. ExerciseIn 30 minutes, as a group, bring back a photo or video of:
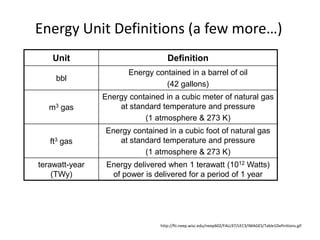
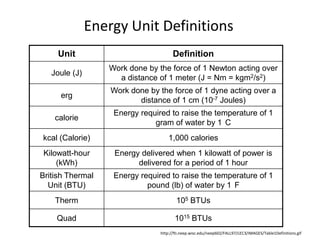
- 5. Energy Unit Definitions (a few more…)http://fti.neep.wisc.edu/neep602/FALL97/LEC3/IMAGES/Table1Definitions.gif
- 6. A BTU?
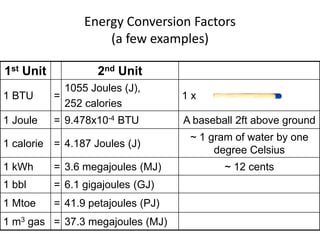
- 7. Energy Conversion Factors (a few examples)
- 8. A cliff bar?= 252 Calories = 252 kilocalories = 1000 BTUs (1 BTU = 252 cal)= 1000
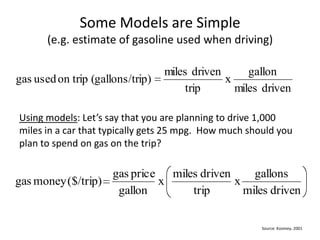
- 10. Some Models are Simple (e.g. estimate of gasoline used when driving)Using models: Let’s say that you are planning to drive 1,000 miles in a car that typically gets 25 mpg. How much should you plan to spend on gas on the trip?Source: Koomey, 2001
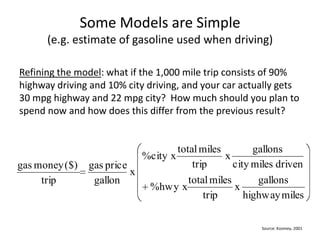
- 11. Some Models are Simple (e.g. estimate of gasoline used when driving)Refining the model: what if the 1,000 mile trip consists of 90% highway driving and 10% city driving, and your car actually gets 30 mpg highway and 22 mpg city? How much should you plan to spend now and how does this differ from the previous result?Source: Koomey, 2001
- 12. Back-of-the-Envelope Estimates(a very powerful tool)How many gallons gasoline are used on average by light duty vehicles (passenger cars, trucks, and SUVs) in the USA each day?
- 13. Unit conversions:Mundane, but critically important for energy analysisPractice makes perfect (but don’t spend all your time memorizing conversion factors)Simple mathematical models and “back of the envelope” calculationsMost energy problems have a quantitative dimension (though numbers are rarely the whole story)
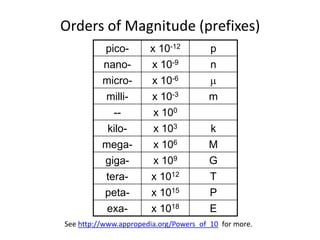
- 14. Orders of Magnitude (prefixes)See http://www.appropedia.org/Powers_of_10 for more.
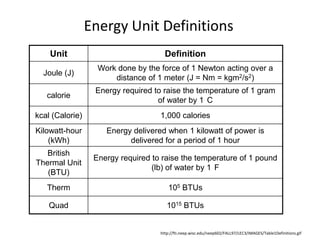
- 15. Energy Unit Definitionshttp://fti.neep.wisc.edu/neep602/FALL97/LEC3/IMAGES/Table1Definitions.gif
- 16. a Joule is thework done to produce one watt continuously for one secondkinetic energy of a 2 kg mass moving at 1 m/s. (given by E = ½mv²)potential energy of a 1 kg mass 1 m high in a gravitational field of 1 m/s². Earth's gravity is 9.81 m/s² at sea level, so 1 kg at 1 m has a potential energy of 9.81 joules.energy required to lift a small apple one meter straight up.amount of heat energy that a quiet person generates every hundredth of a second.energy required to heat one gram of dry, cool air by 1 degree Celsius.
Editor's Notes
- #2: http://www.humboldt.edu/http://www.humboldt.edu/~lrg3http://www.ijsle.org/http://eco-hostel.org/http://dyfference.org/http://appropedia.org/http://humboldt.edu/~ccathttp://green-wheels.org/http://www.thefullbellyproject.org/http://thewaterpod.org/http://www.locally-delicious.org/
- #17: JouleThe work required to move an electric charge of one coulomb through an electrical potential difference of one volt; or one coulomb volt, with the symbol C·V.The work done to produce power of one watt continuously for one second; or one watt second The kinetic energy of a 2 kg mass moving at a velocity of 1 m/sec. The energy is linear in the mass but quadratic in the velocity, being given by E = ½mv².The potential energy of a 1 kg mass at an elevation of 1 m above a reference point in a gravitational field of 1 m/s². Earth's gravity being 9.81 m/s² at sea level, 1 kg at 1 m above the Earth's surface has a potential energy of 9.8 joules relative to that surface. When dropped the potential energy gradually becomes kinetic energy, with the conversion being complete at the instant the mass passes the reference point. Whereas kinetic energy is relative to an inertial frame, in this case that of the earth, potential energy is relative to a position, in this case the Earth's surface.the energy required to lift a small apple one metre straight up.the energy released when that same apple falls one meter to the ground.the amount of energy, as heat, that a quiet person generates every hundredth of a second.the energy required to heat one gram of dry, cool air by 1 degree Celsius.one hundredth of the energy a person can receive by drinking a 1mm diameter drop of beer.the kinetic energy of an adult human moving a distance of about a handspan every second.