Upper Secondary Chemistry-Rust
Download as pptx, pdf0 likes533 views
Acids and salts speed up the rate of rusting by increasing the concentration of ions, allowing more ion movement which causes iron to lose electrons faster and form iron oxide. Rusting can be prevented by applying a barrier like paint, oil, grease or electroplating between the metal and oxygen/water. Electroplating involves placing the plating metal as the anode and the metal to be plated as the cathode in an electrolyte solution. Common metals used for plating include tin, silver, nickel, chromium, zinc and gold.
1 of 10
Download to read offline









Ad
Recommended
Chemistry of iron rusting
Chemistry of iron rustinghowellsc
╠²
Iron rusts through an electrochemical process that requires the presence of water. More acidic water, with a lower pH level, increases corrosion as more hydrogen ions consume electrons to create hydrogen gas instead of water. When iron is exposed to moist outdoor conditions, it begins to rust through this electrochemical reaction between iron, water, and oxygen.metals and rusting of irons
metals and rusting of ironsNur Atiqah M. Hanapiah
╠²
The document discusses an experiment investigating the effects of different metals on the rusting of iron, noting that more electropositive metals prevent rusting while less electropositive metals increase the rate of rusting. Through a series of tests using iron nails and various metals, results showed the presence of blue color indicating iron(II) ions when rusting occurred. The findings support the hypothesis that electropositivity influences the corrosion process of iron.Corrosion
Corrosion projecteciencies
╠²
1) Corrosion is an oxidation reaction that occurs when a metal reacts with oxygen or water, causing the metal atoms to lose electrons and form metal ions.
2) Rusting is the corrosion of iron, which requires water, oxygen, and iron to produce iron ions and hydroxide ions.
3) An experiment was conducted using iron nails attached to magnesium, bare, or attached to copper in a salt solution with an indicator that turns blue in the presence of iron ions to observe their different corrosion rates.Corrosion
CorrosionPadam Banthia
╠²
This document presents a chemistry project on the rusting of iron, detailing the process of corrosion and the effects of various solutions on iron nails. It highlights the oxidation of iron in different environments and includes an experimental setup with sodium chloride and sulfuric acid solutions. The results indicate that sulfuric acid causes the most rusting, followed by sodium chloride solution and water.Corrosion
CorrosionDeepak Kurrey
╠²
1. Galvanic corrosion occurred when copper (Cu) and lead (Pb) metals were in contact where they were attached on the roof of Sampson Hall.
2. When dissimilar metals like Cu and Pb are in contact in the presence of an electrolyte like water (H2O), an electrochemical reaction occurs between them.
3. In the galvanic series, Cu is more noble than Pb, so the Pb acted as the anode and corroded as electrons flowed from it to the Cu cathode.
This caused leaks to form from the breakdown of the Pb materialCorrosion, standard grade chemistry
Corrosion, standard grade chemistryacalero
╠²
The document discusses corrosion and rusting of metals such as iron. It explains that rusting is the corrosion of iron caused by a redox reaction with oxygen and water to form iron(III) oxide. Rusting occurs faster in the presence of salts or acids as they provide electrolytes that allow electrons to transfer between reactants. Various methods are described to prevent corrosion through physical barriers or sacrificial protection of more reactive metals.Rusting Of Iron
Rusting Of Iron yuva raj
╠²
The document discusses corrosion of metals and methods to prevent it. It focuses on the sacrificial anode cathode protection system. It explains that corrosion occurs via electrochemical reactions where the metal acts as the anode and transfers electrons to the cathode. Coupling iron with more electropositive metals like zinc and magnesium prevents rusting by providing preferential sites for the corrosion reactions. The project involves coupling iron nails with zinc, copper and magnesium to observe their effect on rusting. It is concluded that zinc and magnesium prevent rusting by being more electropositive than iron and corroding instead.Redox part 3= rusting - reactivity series and diff between electrolytic cell...
Redox part 3= rusting - reactivity series and diff between electrolytic cell...MRSMPC
╠²
The document discusses the process of rusting, highlighting the chemical reactions involving iron, water, and oxygen that lead to the formation of rust (hydrated iron (III) oxide). It explains the electrochemical principles behind rusting, preventive measures such as protective coatings, and the reactivity series of metals that influence corrosion resistance. Additionally, it includes experimental observations and theoretical explanations for the rusting process and methods of metal extraction.Rusting of fe and ss
Rusting of fe and ssonlinemetallurgy.com
╠²
Iron and steel corrode when exposed to oxygen in the air, forming rust. Rust occurs through a chemical reaction where iron reacts with oxygen to form iron oxide. The iron oxide molecules are larger than the original iron atoms, causing rust to flake off and weaken the metal over time. Some elements added to steel, such as copper, can slow the rusting process by forming protective oxide layers. Stainless steel is an alloy that is highly resistant to rusting due to the formation of a thin, stable chromium oxide film that protects the underlying metal.Chemistry investigatory project on Effect of Metal Coupling on Corrosion
Chemistry investigatory project on Effect of Metal Coupling on CorrosionReshop Nanda
╠²
The document describes an experiment on the corrosion of different metals and metal alloys in acidic and basic solutions. Key findings include:
- Brass and iron corroded most in acid, while brass and zinc corroded most in base. Aluminum corroded least in both.
- Coupling metals affected corrosion rates, with iron-aluminum coupling corroding most in acid and brass-aluminum in base.
- Corrosion is explained by the electrochemical process and metal reactivity based on their reduction potentials.
- Common corrosion protection methods are also outlined.effect of pH level on corrosion rate
effect of pH level on corrosion rateKoredexy
╠²
This experiment investigated the effect of pH levels on corrosion rates of copper, aluminum, and stainless steel. The metals were immersed in solutions with pH levels of 11, 7, and 3. Aluminum showed the highest corrosion rates, which increased with higher pH levels. Copper had no corrosion at pH 7 but equal rates at pH 11 and 3. Stainless steel had consistent low corrosion rates across pH levels. The experiment demonstrated that pH significantly impacts corrosion for different metals, with aluminum being most susceptible.Rusting
RustingPuneetpatel123
╠²
Rusting is the process where iron turns into iron oxide when it comes into contact with water and oxygen. It is a type of corrosion that occurs naturally. The chemical names for rust include iron oxide, ferric oxide, and hematite. The best ways to prevent iron from rusting are to spray paint it, put oil on it, or apply melted wax. Galvanizing, which involves coating iron or steel with a protective layer of zinc, is another effective way to prevent rusting. Other metals that can rust include steel, copper, and bronze.Corrosion std 12 ch 3l RUSTING OF IRON l GALVANIZATION
Corrosion std 12 ch 3l RUSTING OF IRON l GALVANIZATIONMAYURI SOMPURA
╠²
1) Rusting is the corrosion of iron caused by a reaction with oxygen and moisture in the air.
2) The rusting process is electrochemical, where iron acts as the anode and is oxidized, releasing electrons, while oxygen is reduced at the cathode.
3) Factors that affect rusting include moisture content, the purity and type of metal, amount of impurities, and presence of electrolytes that allow conduction of ions involved in the corrosion reaction.Chemistry Project on the effect of metal coupling on the rusting of iron
Chemistry Project on the effect of metal coupling on the rusting of ironSanjay Cr├║z├®
╠²
This document summarizes a student project on the effect of metal coupling on the rusting of iron. The project investigated how coupling iron with more electropositive metals like zinc and magnesium prevents rusting, while coupling with less electropositive metals like copper facilitates rusting. The student presents background on corrosion, describes procedures to couple iron nails with different metals and observe rusting over time, and concludes that iron rusts less when coupled with more electropositive metals.investigation project of chemistry on metal coupling
investigation project of chemistry on metal coupling RAM CHOUDHARY
╠²
This document summarizes a student's investigatory project on the effect of metal coupling on rusting of iron. The student coupled iron nails with zinc, magnesium, copper, and no metal as a control. They observed that coupling iron with the more electropositive metals zinc and magnesium prevented rusting, as seen by the formation of blue patches around the nails. Coupling iron with the less electropositive copper facilitated rusting, as seen by the formation of pink patches. The student concluded that coupling iron with more electropositive metals resists corrosion and rusting, while coupling with less electropositive metals increases rusting.Chemistry project
Chemistry projecthimanshu626
╠²
This document contains a dissertation submitted by Himanshu Kumar on the topic "Effect of Metal Coupling on Rusting of Iron". The 3-page summary includes an introduction, aim, materials, theory, procedure, observations, results, conclusions and bibliography. The student investigated how coupling different metals (iron, aluminum, brass and zinc) affects the rusting rate of iron in acidic and basic solutions. The results showed brass had the highest corrosion rate while aluminum had the lowest. In acidic solutions, iron coupled with aluminum corroded fastest while iron coupled with zinc corroded slowest. In basic solutions, the rates followed the order of brass coupled with aluminum corroding fastest and brass coupled with zinc corroding slowest. The conclusions12th CHEMISTRY PROJECT - RUSTING OF IRON
12th CHEMISTRY PROJECT - RUSTING OF IRONUjjwal Varshney
╠²
This document is a chemistry project report by Utkarsh Varshney of class 12 on the topic of rusting of iron. It includes an introduction to rusting and its mechanisms, experiments conducted on rusting of iron under different conditions, and methods to prevent rusting. The project was completed under the guidance of the teacher Mr. Vikram Bhambhu to fulfill CBSE practical requirements.Corrosion
CorrosionRachit Bhalla
╠²
The document discusses corrosion, which is defined as the deterioration of materials through chemical or electrochemical reactions with the environment. It observes the appearance of five pure metals - iron, silver, copper, lead, and aluminum - and their corroded appearances. The main agents that cause corrosion are listed as acids, alkalies, salts, water, and oxygen. Some methods for preventing corrosion mentioned are galvanization, using electrolytes, and applying sacrificial coatings of more reactive metals.Corrosion
CorrosionKumar
╠²
Corrosion is the deterioration of materials due to chemical or electrochemical reaction with the environment. It is an inevitable process that leads to significant economic losses. Corrosion engineering studies corrosion mechanisms and works to prevent or control corrosion economically and safely. Common types of corrosion include galvanic, erosion, crevice, pitting, and microbiologically influenced corrosion. Factors that influence corrosion include the metal properties, environmental conditions like temperature, pH, and presence of ions. Protection methods include material selection, cathodic protection, modifying the environment, metallic coatings, inorganic coatings, and organic coatings.Project on effect of metal coupling on rusting of iron
Project on effect of metal coupling on rusting of ironEkansh Wagadare
╠²
The document describes an experiment on the effect of metal coupling on the rusting of iron. Four iron nails were used - one each wrapped in zinc, magnesium, and copper strips, and one bare nail. The nails were placed in agar solution and observed after a day. Rusting was prevented on the zinc-wrapped nail but facilitated on the copper-wrapped nail. The magnesium-wrapped nail showed an intermediate level of rusting. The document concludes that coupling iron with more electropositive metals like zinc and magnesium resists rusting, while coupling with less electropositive metals like copper increases rusting.STUDY OF THE EFFECT OF METAL COUPLING ON THE RUSTING OF IRON
STUDY OF THE EFFECT OF METAL COUPLING ON THE RUSTING OF IRONKrishna Yadav
╠²
The document explores the mechanism of metal corrosion, focusing on the rusting of iron and the impact of metal coupling on this process. Key methods to prevent corrosion, such as barrier protection and galvanization, are discussed, along with an experimental procedure to demonstrate these effects. Conclusions indicate that coupling iron with more electropositive metals like zinc or magnesium reduces rusting, while coupling with less electropositive metals like copper increases rusting.Wet or electrochemical corrosion
Wet or electrochemical corrosionSarala Prasanna Pattanaik
╠²
The document provides an in-depth analysis of wet or electrochemical corrosion, detailing its mechanisms, types of cathodic reactions, and the distinction between dry and wet corrosion. It elaborates on the processes of oxidation and reduction involving metals in various solutions, emphasizing the factors influencing corrosion such as the presence of electrolytes and differences in aeration. Additionally, it outlines examples of corrosion types and compares the characteristics of dry and wet corrosion.Investigatory chemistry
Investigatory chemistryRADO7900
╠²
This document presents a student project on the effect of metal coupling on iron rusting. The student Mayank Chaudhary from JKG International School studied how coupling iron with more electropositive metals like zinc and magnesium prevents rusting, while coupling with less electropositive copper facilitates rusting. Through experimentation with iron nails wrapped in different metals, observation of color changes, and analysis, the student was able to conclude that metal coupling affects iron's corrosion, with more positive metals protecting iron and less positive metals increasing rusting.Corrosion and degradation of materials
Corrosion and degradation of materialsGhassan Alshahiri
╠²
This document defines corrosion as the deterioration of a material due to reaction with its environment, especially oxygen. Corrosion is an electrochemical process that occurs when a metal is exposed to oxygen and an electrolyte like water. Three factors are required for corrosion: a metal, oxygen, and an electrolyte. Corrosion causes deterioration of manufactured products and infrastructure. Understanding and preventing corrosion is important for maintaining machinery and structures. Corrosion occurs through oxidation and reduction reactions and can be localized or generalized. Methods to prevent corrosion include painting, sacrificial anodes, cathodic protection, and passivation.Corrosion session 11 july 14
Corrosion session 11 july 14Amol Pawar
╠²
1. Corrosion is an electrochemical process involving oxidation and reduction reactions. It requires an anode, cathode, electrolyte, and an electrically conducting path.
2. At the anode, iron oxidizes to ferrous ions which then react with hydroxyl ions from the cathode to form iron hydroxide and iron oxide. The products occupy more volume than the original steel causing stresses in the concrete.
3. Chlorides from deicing salts or seawater can destroy the protective oxide layer and accelerate corrosion. Carbonation reduces concrete's alkalinity allowing the protective layer to break down.CORROSION (Mechanism of Oxygen absorption in alkaline medium)
CORROSION (Mechanism of Oxygen absorption in alkaline medium)As Win
╠²
Corrosion is the destruction or deterioration of a metal due to chemical or electrochemical reaction with its environment. There are two main types of corrosion depending on the medium: dry corrosion explained by chemical theory and wet corrosion explained by electrochemical theory. Rusting of iron occurs when it is exposed to moist air or liquid and involves an oxidation-reduction process where iron oxidizes to iron ions at the anode and oxygen is reduced to hydroxide ions at the cathode. Similarly, in alkaline medium, small scratches on iron act as anodes for the oxidation of iron while the rest of the surface acts as the cathode for the reduction of oxygen to hydroxide ions. White rust on zinc occurs when zinc is exposed toChemistry project
Chemistry projectafnaanqureshi1
╠²
This document is a student project on studying the effect of metal coupling on rusting of iron. It includes an introduction on corrosion, the electrochemical mechanism of rusting, and common prevention methods. The aim is to investigate how coupling iron with different metals affects rusting. The procedure involves coupling iron nails with zinc, copper, or magnesium and observing any rust formation. The results showed that coupling with more electropositive metals like zinc and magnesium prevented rusting, while coupling with less electropositive copper facilitated rusting.analysis of corrosion ~dk
analysis of corrosion ~dkDinesh Kumar Meena
╠²
The document describes an experiment studying the effect of metal coupling on corrosion rates in acidic and basic solutions. It finds that brass has the highest corrosion rate while aluminum has the lowest, and that corrosion rates generally decrease when metals are coupled compared to individually. The purpose is to better understand corrosion of common metals and alloys in order to reduce costs from replacement of corroded materials.Corrosion Fall 21 Chemistry 2023 fall
Corrosion Fall 21 Chemistry 2023 fallMUHAMMADAHMAD27172
╠²
The document discusses the electrochemical processes involved in the corrosion of iron, specifically how rust (Fe2O3.xH2O) forms in various environments due to factors like acidity and dissolved salts. It outlines both the reactions leading to corrosion and strategies for preventing it, such as coatings, passivation, and the use of sacrificial anodes like magnesium. Additionally, it highlights the effectiveness of alloying and specific paints in reducing corrosion rates.Investigatory project Chemistry.docx
Investigatory project Chemistry.docxDheebannambishwar
╠²
The document discusses the corrosion and rusting of metals like iron. It explains that rusting is an electrochemical process where iron loses electrons and oxygen gains electrons, forming hydroxide ions. The rate of corrosion is affected by water and electrolytes. Different metals coupled to an iron nail will affect its rusting - a more electropositive metal like zinc will prevent rusting, while a less electropositive metal like magnesium will facilitate rusting. The document also outlines the reactions involved in rust formation and discusses how rust changes over time based on conditions.More Related Content
What's hot (20)
Rusting of fe and ss
Rusting of fe and ssonlinemetallurgy.com
╠²
Iron and steel corrode when exposed to oxygen in the air, forming rust. Rust occurs through a chemical reaction where iron reacts with oxygen to form iron oxide. The iron oxide molecules are larger than the original iron atoms, causing rust to flake off and weaken the metal over time. Some elements added to steel, such as copper, can slow the rusting process by forming protective oxide layers. Stainless steel is an alloy that is highly resistant to rusting due to the formation of a thin, stable chromium oxide film that protects the underlying metal.Chemistry investigatory project on Effect of Metal Coupling on Corrosion
Chemistry investigatory project on Effect of Metal Coupling on CorrosionReshop Nanda
╠²
The document describes an experiment on the corrosion of different metals and metal alloys in acidic and basic solutions. Key findings include:
- Brass and iron corroded most in acid, while brass and zinc corroded most in base. Aluminum corroded least in both.
- Coupling metals affected corrosion rates, with iron-aluminum coupling corroding most in acid and brass-aluminum in base.
- Corrosion is explained by the electrochemical process and metal reactivity based on their reduction potentials.
- Common corrosion protection methods are also outlined.effect of pH level on corrosion rate
effect of pH level on corrosion rateKoredexy
╠²
This experiment investigated the effect of pH levels on corrosion rates of copper, aluminum, and stainless steel. The metals were immersed in solutions with pH levels of 11, 7, and 3. Aluminum showed the highest corrosion rates, which increased with higher pH levels. Copper had no corrosion at pH 7 but equal rates at pH 11 and 3. Stainless steel had consistent low corrosion rates across pH levels. The experiment demonstrated that pH significantly impacts corrosion for different metals, with aluminum being most susceptible.Rusting
RustingPuneetpatel123
╠²
Rusting is the process where iron turns into iron oxide when it comes into contact with water and oxygen. It is a type of corrosion that occurs naturally. The chemical names for rust include iron oxide, ferric oxide, and hematite. The best ways to prevent iron from rusting are to spray paint it, put oil on it, or apply melted wax. Galvanizing, which involves coating iron or steel with a protective layer of zinc, is another effective way to prevent rusting. Other metals that can rust include steel, copper, and bronze.Corrosion std 12 ch 3l RUSTING OF IRON l GALVANIZATION
Corrosion std 12 ch 3l RUSTING OF IRON l GALVANIZATIONMAYURI SOMPURA
╠²
1) Rusting is the corrosion of iron caused by a reaction with oxygen and moisture in the air.
2) The rusting process is electrochemical, where iron acts as the anode and is oxidized, releasing electrons, while oxygen is reduced at the cathode.
3) Factors that affect rusting include moisture content, the purity and type of metal, amount of impurities, and presence of electrolytes that allow conduction of ions involved in the corrosion reaction.Chemistry Project on the effect of metal coupling on the rusting of iron
Chemistry Project on the effect of metal coupling on the rusting of ironSanjay Cr├║z├®
╠²
This document summarizes a student project on the effect of metal coupling on the rusting of iron. The project investigated how coupling iron with more electropositive metals like zinc and magnesium prevents rusting, while coupling with less electropositive metals like copper facilitates rusting. The student presents background on corrosion, describes procedures to couple iron nails with different metals and observe rusting over time, and concludes that iron rusts less when coupled with more electropositive metals.investigation project of chemistry on metal coupling
investigation project of chemistry on metal coupling RAM CHOUDHARY
╠²
This document summarizes a student's investigatory project on the effect of metal coupling on rusting of iron. The student coupled iron nails with zinc, magnesium, copper, and no metal as a control. They observed that coupling iron with the more electropositive metals zinc and magnesium prevented rusting, as seen by the formation of blue patches around the nails. Coupling iron with the less electropositive copper facilitated rusting, as seen by the formation of pink patches. The student concluded that coupling iron with more electropositive metals resists corrosion and rusting, while coupling with less electropositive metals increases rusting.Chemistry project
Chemistry projecthimanshu626
╠²
This document contains a dissertation submitted by Himanshu Kumar on the topic "Effect of Metal Coupling on Rusting of Iron". The 3-page summary includes an introduction, aim, materials, theory, procedure, observations, results, conclusions and bibliography. The student investigated how coupling different metals (iron, aluminum, brass and zinc) affects the rusting rate of iron in acidic and basic solutions. The results showed brass had the highest corrosion rate while aluminum had the lowest. In acidic solutions, iron coupled with aluminum corroded fastest while iron coupled with zinc corroded slowest. In basic solutions, the rates followed the order of brass coupled with aluminum corroding fastest and brass coupled with zinc corroding slowest. The conclusions12th CHEMISTRY PROJECT - RUSTING OF IRON
12th CHEMISTRY PROJECT - RUSTING OF IRONUjjwal Varshney
╠²
This document is a chemistry project report by Utkarsh Varshney of class 12 on the topic of rusting of iron. It includes an introduction to rusting and its mechanisms, experiments conducted on rusting of iron under different conditions, and methods to prevent rusting. The project was completed under the guidance of the teacher Mr. Vikram Bhambhu to fulfill CBSE practical requirements.Corrosion
CorrosionRachit Bhalla
╠²
The document discusses corrosion, which is defined as the deterioration of materials through chemical or electrochemical reactions with the environment. It observes the appearance of five pure metals - iron, silver, copper, lead, and aluminum - and their corroded appearances. The main agents that cause corrosion are listed as acids, alkalies, salts, water, and oxygen. Some methods for preventing corrosion mentioned are galvanization, using electrolytes, and applying sacrificial coatings of more reactive metals.Corrosion
CorrosionKumar
╠²
Corrosion is the deterioration of materials due to chemical or electrochemical reaction with the environment. It is an inevitable process that leads to significant economic losses. Corrosion engineering studies corrosion mechanisms and works to prevent or control corrosion economically and safely. Common types of corrosion include galvanic, erosion, crevice, pitting, and microbiologically influenced corrosion. Factors that influence corrosion include the metal properties, environmental conditions like temperature, pH, and presence of ions. Protection methods include material selection, cathodic protection, modifying the environment, metallic coatings, inorganic coatings, and organic coatings.Project on effect of metal coupling on rusting of iron
Project on effect of metal coupling on rusting of ironEkansh Wagadare
╠²
The document describes an experiment on the effect of metal coupling on the rusting of iron. Four iron nails were used - one each wrapped in zinc, magnesium, and copper strips, and one bare nail. The nails were placed in agar solution and observed after a day. Rusting was prevented on the zinc-wrapped nail but facilitated on the copper-wrapped nail. The magnesium-wrapped nail showed an intermediate level of rusting. The document concludes that coupling iron with more electropositive metals like zinc and magnesium resists rusting, while coupling with less electropositive metals like copper increases rusting.STUDY OF THE EFFECT OF METAL COUPLING ON THE RUSTING OF IRON
STUDY OF THE EFFECT OF METAL COUPLING ON THE RUSTING OF IRONKrishna Yadav
╠²
The document explores the mechanism of metal corrosion, focusing on the rusting of iron and the impact of metal coupling on this process. Key methods to prevent corrosion, such as barrier protection and galvanization, are discussed, along with an experimental procedure to demonstrate these effects. Conclusions indicate that coupling iron with more electropositive metals like zinc or magnesium reduces rusting, while coupling with less electropositive metals like copper increases rusting.Wet or electrochemical corrosion
Wet or electrochemical corrosionSarala Prasanna Pattanaik
╠²
The document provides an in-depth analysis of wet or electrochemical corrosion, detailing its mechanisms, types of cathodic reactions, and the distinction between dry and wet corrosion. It elaborates on the processes of oxidation and reduction involving metals in various solutions, emphasizing the factors influencing corrosion such as the presence of electrolytes and differences in aeration. Additionally, it outlines examples of corrosion types and compares the characteristics of dry and wet corrosion.Investigatory chemistry
Investigatory chemistryRADO7900
╠²
This document presents a student project on the effect of metal coupling on iron rusting. The student Mayank Chaudhary from JKG International School studied how coupling iron with more electropositive metals like zinc and magnesium prevents rusting, while coupling with less electropositive copper facilitates rusting. Through experimentation with iron nails wrapped in different metals, observation of color changes, and analysis, the student was able to conclude that metal coupling affects iron's corrosion, with more positive metals protecting iron and less positive metals increasing rusting.Corrosion and degradation of materials
Corrosion and degradation of materialsGhassan Alshahiri
╠²
This document defines corrosion as the deterioration of a material due to reaction with its environment, especially oxygen. Corrosion is an electrochemical process that occurs when a metal is exposed to oxygen and an electrolyte like water. Three factors are required for corrosion: a metal, oxygen, and an electrolyte. Corrosion causes deterioration of manufactured products and infrastructure. Understanding and preventing corrosion is important for maintaining machinery and structures. Corrosion occurs through oxidation and reduction reactions and can be localized or generalized. Methods to prevent corrosion include painting, sacrificial anodes, cathodic protection, and passivation.Corrosion session 11 july 14
Corrosion session 11 july 14Amol Pawar
╠²
1. Corrosion is an electrochemical process involving oxidation and reduction reactions. It requires an anode, cathode, electrolyte, and an electrically conducting path.
2. At the anode, iron oxidizes to ferrous ions which then react with hydroxyl ions from the cathode to form iron hydroxide and iron oxide. The products occupy more volume than the original steel causing stresses in the concrete.
3. Chlorides from deicing salts or seawater can destroy the protective oxide layer and accelerate corrosion. Carbonation reduces concrete's alkalinity allowing the protective layer to break down.CORROSION (Mechanism of Oxygen absorption in alkaline medium)
CORROSION (Mechanism of Oxygen absorption in alkaline medium)As Win
╠²
Corrosion is the destruction or deterioration of a metal due to chemical or electrochemical reaction with its environment. There are two main types of corrosion depending on the medium: dry corrosion explained by chemical theory and wet corrosion explained by electrochemical theory. Rusting of iron occurs when it is exposed to moist air or liquid and involves an oxidation-reduction process where iron oxidizes to iron ions at the anode and oxygen is reduced to hydroxide ions at the cathode. Similarly, in alkaline medium, small scratches on iron act as anodes for the oxidation of iron while the rest of the surface acts as the cathode for the reduction of oxygen to hydroxide ions. White rust on zinc occurs when zinc is exposed toChemistry project
Chemistry projectafnaanqureshi1
╠²
This document is a student project on studying the effect of metal coupling on rusting of iron. It includes an introduction on corrosion, the electrochemical mechanism of rusting, and common prevention methods. The aim is to investigate how coupling iron with different metals affects rusting. The procedure involves coupling iron nails with zinc, copper, or magnesium and observing any rust formation. The results showed that coupling with more electropositive metals like zinc and magnesium prevented rusting, while coupling with less electropositive copper facilitated rusting.analysis of corrosion ~dk
analysis of corrosion ~dkDinesh Kumar Meena
╠²
The document describes an experiment studying the effect of metal coupling on corrosion rates in acidic and basic solutions. It finds that brass has the highest corrosion rate while aluminum has the lowest, and that corrosion rates generally decrease when metals are coupled compared to individually. The purpose is to better understand corrosion of common metals and alloys in order to reduce costs from replacement of corroded materials.Similar to Upper Secondary Chemistry-Rust (20)
Corrosion Fall 21 Chemistry 2023 fall
Corrosion Fall 21 Chemistry 2023 fallMUHAMMADAHMAD27172
╠²
The document discusses the electrochemical processes involved in the corrosion of iron, specifically how rust (Fe2O3.xH2O) forms in various environments due to factors like acidity and dissolved salts. It outlines both the reactions leading to corrosion and strategies for preventing it, such as coatings, passivation, and the use of sacrificial anodes like magnesium. Additionally, it highlights the effectiveness of alloying and specific paints in reducing corrosion rates.Investigatory project Chemistry.docx
Investigatory project Chemistry.docxDheebannambishwar
╠²
The document discusses the corrosion and rusting of metals like iron. It explains that rusting is an electrochemical process where iron loses electrons and oxygen gains electrons, forming hydroxide ions. The rate of corrosion is affected by water and electrolytes. Different metals coupled to an iron nail will affect its rusting - a more electropositive metal like zinc will prevent rusting, while a less electropositive metal like magnesium will facilitate rusting. The document also outlines the reactions involved in rust formation and discusses how rust changes over time based on conditions.Factors affecting corrosion & control measures
Factors affecting corrosion & control measuresSarala Prasanna Pattanaik
╠²
The document discusses the mechanisms, types, and factors influencing corrosion, including both electrochemical and chemical processes. It outlines various methods for protecting against corrosion, such as barrier, sacrificial, and cathodic protection, along with the use of coatings and alloys to enhance metal resistance. Additionally, it details the roles of environmental factors and metal purity in corrosion rates, along with specific inhibitors that can mitigate corrosion processes.Factors affecting and prevention_Corrosion (1).ppt
Factors affecting and prevention_Corrosion (1).pptToranSahu18
╠²
The document discusses various factors influencing corrosion, including the nature of metals and their environments, such as purity, physical state, temperature, humidity, pH, and electrolyte nature. It examines mechanisms of corrosion and protective measures like barrier protection, sacrificial protection, alloy formation, and cathodic protection. Additionally, the document outlines methods to control and prevent corrosion, emphasizing the importance of material selection, equipment design, and use of inhibitors.Grade 11 rusting and recycling
Grade 11 rusting and recyclingNellexo
╠²
The document provides information on topics related to rusting and corrosion of iron for CXC exams, including:
- Rusting is the oxidation of iron in the presence of oxygen and water to form hydrated iron(III) oxide. Only iron and alloys containing iron rust.
- Methods for preventing rust include using protective coatings like paint and grease, attaching a more reactive sacrificial metal like zinc, and creating rust-resistant alloys like stainless steel.
- Recycling metals has economic, social, and environmental benefits like reducing costs and waste, conserving resources, and decreasing pollution from metal extraction. However, recycling also has expenses and implementation challenges.Corrosion
CorrosionSwapnil Bhele
╠²
Corrosion occurs when a metal loses electrons and forms positive metal ions, reacting with oxygen and water. For iron, this forms rust. Corrosion happens faster in the presence of salt, pollutants, and heat, as they help carry electrons away from the metal. Common prevention methods include barriers like painting or greasing, sacrificial protection using more reactive metals, cathodic protection with batteries, and coating metals like galvanizing or electroplating.Physical and chemical changes
Physical and chemical changesShauryaSingg
╠²
This document discusses physical and chemical changes. It defines physical changes as changes where a substance undergoes change without forming a new substance, and chemical changes as changes where new substances are formed. It provides examples like heating water and melting butter as physical changes, and cooking food and lighting a match as chemical changes. The document also discusses the rusting of iron as a chemical change, outlining factors that affect rusting like moisture, acid, salt, and impurities. It describes galvanization and using stainless steel as ways to prevent rusting of iron. Finally, it briefly discusses crystallization as a physical change involving deriving crystals from solutions.Corrosion of constructional steels in marine and industrial environment
Corrosion of constructional steels in marine and industrial environmentSpringer
╠²
The document summarizes corrosion processes of iron and steel. It discusses how iron forms a protective oxide film when exposed to air that inhibits further corrosion. For steel, alloying elements like copper, chromium, and nickel are discussed as improving corrosion resistance by forming a more compact rust scale. Weathering steels are described as developing a tightly adhering rust layer over time from alloying elements like chromium and copper that protects the steel underneath. The document outlines various forms of corrosion and factors affecting corrosion rates of steel in atmospheric environments.Corrosion of constructional steels in marine and industrial environment
Corrosion of constructional steels in marine and industrial environmentSpringer
╠²
The document summarizes corrosion processes of iron and steel. It discusses how iron forms a protective oxide film when exposed to air that inhibits further corrosion. For steel, alloying elements like copper, chromium, and nickel are discussed as improving corrosion resistance by forming a more compact rust scale. Weathering steels are described as developing a tightly adhering rust layer over time from alloying elements like chromium and copper that protects the steel underneath. The document outlines various forms of corrosion and factors affecting corrosion rates of steel in atmospheric environments.Protection of metals from corrosion
Protection of metals from corrosionAwais Chaudhary
╠²
This document discusses various methods for protecting metals from corrosion. It first defines corrosion as the deterioration of materials through chemical interaction with the environment. It then explains that oxygen, humidity, and chemical salts are common causes of corrosion. The document goes on to describe several protection methods, including barrier protection using paints, oils, or electroplating; sacrificial protection using more reactive metals; and cathodic protection of underground pipes using more electropositive anodes.A brief Description of Metal Corrosion and Prevention
A brief Description of Metal Corrosion and PreventionChandran Udumbasseri
╠²
This document gives a brief description on metal corrosion. It envelopes, metal oxidation states, basic electrocheistry for corrion, fundamentals of corrosion, types of corrosion,corrosion prevention methods, corrosion measurement methods, and corrosion inhibitor preparation. trabalho sobre Why do things rust ingles
trabalho sobre Why do things rust inglesSandraReginaPedrosa
╠²
Rust is primarily caused by moisture, oxygen, temperature, impurities, and the pH of the environment, with high humidity and contaminants accelerating oxidation. Preventative measures against rust include applying coatings, galvanization, regular maintenance, and using corrosion-resistant materials. Effective strategies to reduce rust formation also involve moisture control, using rust inhibitors, and employing cathodic protection systems.CORROSION.pptx
CORROSION.pptxPRAVEENKG20PHD0320
╠²
Corrosion is the deterioration of materials due to chemical reactions with their environment. It can occur due to factors like humidity, corrosive gases, stress, electrical currents, and bacteria. There are two main types of corrosion: dry/chemical corrosion which involves direct chemical reactions, and wet/electrochemical corrosion which involves the formation of anodic and cathodic areas on a metal surface. Common corrosion prevention methods include using surface coatings, galvanization, alloyed steels, cathodic protection, and new solutions like EonCoat which provides a maintenance-free protective layer.Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))
Corrosion and Its Types (Basic Chemistry - B.Tech / B.E. ))Afzal Imam
╠²
Corrosion is the spontaneous disintegration of metals due to chemical or electrochemical reactions with the environment, causing the formation of compounds like oxides. It occurs as metals strive to return to stable states from higher energy levels, forming oxide films that can be protective or porous. Various methods exist to control corrosion, including proper design, material selection, environmental modifications, and the use of inhibitors.Corrosion-Dr. Surendran Parambadath
Corrosion-Dr. Surendran ParambadathSurendran Parambadath
╠²
This document discusses corrosion and how it affects metals. It defines corrosion as the slow process of decay of metals due to chemical or electrochemical reaction with their environment. This causes the formation of compounds like oxides on the metal surface. Corrosion occurs through both dry chemical reactions and wet electrochemical reactions that set up galvanic cells. The rate of corrosion is affected by factors like the metal's position in the galvanic series, purity, environment, and characteristics of the corrosion products formed. Common types of corrosion discussed are rusting of iron and methods to prevent or reduce corrosion like coatings, cathodic protection, and modifying the environment.Protection of material from the corrosion
Protection of material from the corrosionMayurMarvaniya1
╠²
This document discusses corrosion, its causes, types, and methods of prevention. Corrosion is the degradation of materials through reaction with their environment. Common types include uniform corrosion, galvanic corrosion, and pitting corrosion. Prevention methods include using sacrificial materials, coating surfaces with primers or barrier coatings, cathodic protection like galvanization or impressed current, and regular maintenance. Understanding corrosion allows better material selection and protection to reduce economic losses.Eight forms of corrosion
Eight forms of corrosionGulfam Hussain
╠²
The document summarizes the 8 main forms of corrosion that can occur in metals. It begins by explaining that corrosion is a natural process that converts refined metals back into more stable forms, driven by thermodynamics. It then describes the key elements that form an electrochemical corrosion cell and discusses various factors that influence corrosion rates. The main types of corrosion covered are uniform corrosion, localized corrosion (including galvanic and crevice corrosion), and stress corrosion cracking. Visual examples of each type of corrosion are provided.Corrosion btech first year detail pdf unit 3 .pptx
Corrosion btech first year detail pdf unit 3 .pptxrajputansh2006
╠²
Corrosion is a destructive chemical and electrochemical reaction between metals and their environments, affecting properties like thickness and conductivity. It can occur through various mechanisms including chemical (dry) and electrochemical (wet) corrosion, influenced by factors like metal nature, environmental conditions, and the presence of impurities. Types of corrosion include galvanic, concentration cell, pitting, and crevice corrosion, each with specific causes, effects, and prevention methods.Investigatory project CHEMISTRY.pdf
Investigatory project CHEMISTRY.pdfDheebannambishwar
╠²
The document describes an experiment on the effect of metal coupling on the rusting of iron. Four iron nails were used - one coupled with zinc, one with magnesium, one with copper, and one alone. The nails were embedded in agar solution and observed after one day. Rusting was prevented on the nail coupled with zinc, the most electropositive metal. Rusting increased on the nail coupled with copper, the least electropositive metal. Coupling iron with more electropositive metals resists rusting, while coupling with less electropositive metals facilitates rusting.Chemistry Investigatory Project Class 12
Chemistry Investigatory Project Class 12Rushil Aggarwal
╠²
This document is a project report by Rushil Aggarwal on the investigation of metal coupling's effect on the rusting of iron, conducted under the guidance of Mr. Anit Rana during the academic year 2015-2016. It details the electrochemical mechanism of rusting, methods to prevent corrosion, and presents experimental observations that illustrate how coupling iron with electropositive metals like zinc and magnesium prevents rusting, while coupling with less electropositive metals like copper facilitates it. The project concludes that proper coupling can significantly enhance the longevity of iron by reducing corrosion.Ad
More from MarioSonic54 (20)
Upper Secondary Social Studies-Regional Relations
Upper Secondary Social Studies-Regional RelationsMarioSonic54
╠²
Regional relations can be established through associations of countries in a region. In 1967, five Southeast Asian countries formed the Association of Southeast Asian Nations (ASEAN) to promote economic, social, cultural and political cooperation. ASEAN aims to protect regional peace and stability and provide opportunities for member countries to resolve disputes peacefully. It now has ten member countries and represents a united voice on international issues.Upper Secondary Geography-Climatic types
Upper Secondary Geography-Climatic typesMarioSonic54
╠²
The document describes three main climatic types: equatorial, monsoon, and cool temperate. Equatorial climate is located between 10┬░N and 10┬░S and is characterized by high temperatures, small temperature ranges, high humidity, heavy rainfall, and no distinct wet or dry seasons. Monsoon climate is located between 5┬░N-25┬░N and 5┬░S-25┬░S and has warmer temperatures than equatorial climates due to a less direct sun angle. Cool temperate climate occurs between 45┬░-60┬░N and 45┬░-60┬░S. Examples of locations for each climate type are provided.Upper Secondary Geography-Groups of people affecting tourism
Upper Secondary Geography-Groups of people affecting tourismMarioSonic54
╠²
There are four main groups that can influence tourism: governments, media, international organizations, and travel writers. Governments have power over visitors by controlling entry/exit policies and developing tourism infrastructure. Media coverage of a location can encourage or discourage visitors by reporting positively or negatively on conditions. International bodies like the WHO provide advisories that impact travel. And travel writers influence readers through first-hand accounts and recommendations in publications and online.Upper Secondary Principles of Account-Profit or loss account
Upper Secondary Principles of Account-Profit or loss accountMarioSonic54
╠²
This document provides an example of how to prepare an income statement or trading account from a trial balance. It shows the sales revenue, cost of sales, gross profit, other income, other expenses, and profit for the period sections of the income statement. Other income is added to gross profit. Other expenses are then deducted to arrive at the profit for the period.Upper Secondary Chemistry-Atomic structure of group i
Upper Secondary Chemistry-Atomic structure of group iMarioSonic54
╠²
This document lists 6 chemical elements in the alkali metal group of the periodic table. It includes lithium, sodium, potassium, rubidium, caesium, and francium.English-Continuous Tense
English-Continuous TenseMarioSonic54
╠²
The document discusses the present continuous, past continuous, and future continuous tenses. The present continuous describes an action happening now that may be temporary. The past continuous describes actions that occurred over a period of time in the past. The future continuous describes a long action in the future that will be interrupted by a shorter action.Upper Secondary Physics-Speed-Time Graph
Upper Secondary Physics-Speed-Time GraphMarioSonic54
╠²
This document describes different types of motion on a speed-time graph. It shows an object at rest, moving at a uniform speed, with uniform acceleration and deceleration, and with non-uniform acceleration and deceleration. The graph illustrates an object's changing speed over time under different conditions of motion.English-Simple Tense
English-Simple TenseMarioSonic54
╠²
This document discusses the simple tenses in English grammar - simple present, simple past, and simple future. The simple present tense is used for recurring or habitual actions, facts, future plans, and present thoughts/feelings. The simple past tense refers to specific or ongoing events/situations that occurred in the past. The simple future tense uses "will" to express voluntary actions, promises, and predictions, and "going to" to talk about plans.Upper Secondary Physics-Distance-Time Graph
Upper Secondary Physics-Distance-Time GraphMarioSonic54
╠²
The document outlines four scenarios involving an object's motion over time: an object at rest, an object moving at a uniform speed of 5 meters per second, an object accelerating, and an object decelerating.Secondary 2 History-Singapore Tackling Challenges of Defense and Security
Secondary 2 History-Singapore Tackling Challenges of Defense and SecurityMarioSonic54
╠²
This document discusses Singapore's challenges with defense and security after gaining independence from Britain in 1971. It outlines how Singapore lacked experience building a strong military and faced a lack of public support for national service. To address these issues, the government established the Ministry of Interior and Defense, passed legislation in 1967 requiring all men to complete national service, and set up training programs and community outreach to gain support. These efforts helped Singapore develop the citizen's army model and strengthen its defense capabilities through cooperation with foreign partners.Secondary 2 Geography-Measures to Reduce Ozone Depletion
Secondary 2 Geography-Measures to Reduce Ozone DepletionMarioSonic54
╠²
The document outlines individual, national, and international efforts to reduce ozone depletion. It recommends that individuals avoid chlorofluorocarbon products, governments ban chlorofluorocarbon and encourage alternatives, and internationally the 1987 Montreal Protocol aimed to reduce then eliminate chlorofluorocarbon use by 2000 as agreed by the US and Europe.Secondary 2 Geography-Ozone Depletion
Secondary 2 Geography-Ozone DepletionMarioSonic54
╠²
The document discusses ozone depletion and its causes and consequences. It explains that ozone in the stratosphere protects the Earth from ultraviolet radiation, but chlorofluorocarbons released into the atmosphere break down into chlorine which destroys ozone molecules. This causes the ozone layer to thin, leaving it unable to fully screen ultraviolet rays. If the ozone layer is depleted faster than it can recover, an "ozone hole" forms. The thinning ozone allows more ultraviolet radiation to reach the Earth's surface, increasing health and environmental risks like skin cancer and disruption of marine and terrestrial ecosystems.Secondary 2 Geography-Global Warming
Secondary 2 Geography-Global WarmingMarioSonic54
╠²
Global warming is caused by increases in greenhouse gases such as carbon dioxide, methane, nitrous oxide, and chlorofluorocarbons being released into the atmosphere from human activities like burning fossil fuels and deforestation. This has led to a 0.5┬░C increase in average global temperatures over the past 100 years. Consequences of global warming include rising sea levels from melting ice caps which threatens coastal populations, increased transmission of infectious diseases by insects, mass extinction of animal species unable to adapt, and disruption of weather patterns causing more extreme events like droughts and heat waves.Secondary 2 History-Singapore Separating From Malaysia
Secondary 2 History-Singapore Separating From MalaysiaMarioSonic54
╠²
Singapore separated from Malaysia in 1965 for both economic and political reasons. Economically, Malaysia imposed tariffs on Singapore's goods and treated Singapore as an economic rival. Politically, the two countries had different approaches to forming political parties along racial lines versus being multiracial. Racial tensions rose and riots broke out in 1964. The Prime Minister of Malaysia decided that separation was the best solution to prevent further conflict. On August 9, 1965, Lee Kuan Yew announced that Singapore had gained independence after separating from Malaysia.Secondary 2 History-Singapore Merging With Malaya
Secondary 2 History-Singapore Merging With MalayaMarioSonic54
╠²
Singapore wanted to merge with Malaya in the 1960s for economic and political reasons. Economically, Singapore lacked natural resources and needed access to Malaya's markets. Politically, merging could help counter communist influence and allow Singapore to gain independence. However, Malaya initially opposed merging due to concerns over the racial balance. After a pro-communist candidate won a 1961 by-election in Singapore, Malaya agreed to allow the merger if Singapore developed Sabah and Sarawak. The merger was approved by citizens and Malaysia was formed in 1963, but Singapore separated in 1965.Ad
Upper Secondary Chemistry-Rust
- 5. Why does acids or salt speed up the rate of rusting? ŌĆó With acids or salt, the concentration of ions increases. This allows more ion movements which will cause the iron to lose electrons faster to form Iron(III) Oxide. ŌĆó This speeds up the rate of rusting
- 6. Prevention of rusting ŌĆó Rusting can be prevented by putting a barrier around the metal ŌĆó Paint, oil, grease, plastic coating and electroplating can be a barrier for the metal
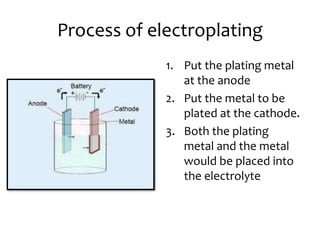
- 8. Process of electroplating 1. Put the plating metal at the anode 2. Put the metal to be plated at the cathode. 3. Both the plating metal and the metal would be placed into the electrolyte
- 9. Common metals used for plating objects ŌĆó Tin ŌĆó Silver ŌĆó Nickel ŌĆó Chromium ŌĆó Zinc ŌĆó Gold
- 10. ŌĆó Tin is commonly used to coat food containers ŌĆó Iron rusts faster when the tin metal coating is damaged because iron is more reactive than tin, therefore, the iron lose electrons faster to form ions which cause rusting to occur faster
