Adhesion Forces during Coagulation as Evaluated by Atomic Force Microscopy
- 1. Thesis Defense By Ajay Kashi Graduate Student, Civil & Environmental Engineering Department
- 2. Overview Introduction to coagulation Objective of research work Introduction to Atomic Force Microscope (AFM) Experimental results and discussion Conclusion and future work
- 3. Coagulation Addition of chemicals to water for increasing the tendency of smaller particles to attach to one another and effect their removal by precipitation Chemistry of Coagulation is based on Types of particles Particle Stability & Surface Charge on particles
- 4. Concept of Electric Double Layer In natural waters, particles are predominantly –vely charged. Existence of two layers of ions over the surface of particles. Zeta Potential is the potential gradient over diffuse layer. Zeta Potential has a maximum value at the surface & decreases with distance. Addition of an electrolyte decreases the double layer thickness and hence zeta potential. This decreases repulsive forces and van der waals forces dominate resulting in coagulation.
- 5. Contd…… Aggregation effect increases greatly with the valence of electrolyte. Hence Tri-valent cations (Al 3+ & Fe 3+ ) are primarily used for coagulation. Coagulants used in water treatment are Ferric Chloride, Ferric Sulfate, Sodium Aluminate and Aluminum Sulfate. Commonly used coagulants are, Aluminum Sulfate (Alum) and Ferric Chloride.
- 6. Jar Tests Jar tests are conducted to determine optimum coagulant dosages for the removal of particulate matter. Results are based on rate of agglomeration, settleability of flocs & clarity of supernatant water.
- 7. Objectives Use Atomic Force microscopy (AFM) to directly measure the forces of interaction between biological particles during coagulation. Correlate force measurements with real time coagulation studies. Develop basic understanding of the interaction forces to Evaluate Bacterial Adhesion during Coagulation.
- 8. Advantages of AFM Technique Currently the only technique to measure interactions between bacteria and colloidal particles. Sensitive enough to detect forces in the nN range. All measurements are carried out in a physiological buffer solution.
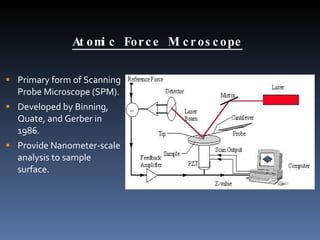
- 9. Atomic Force Microscope Primary form of Scanning Probe Microscope (SPM). Developed by Binning, Quate, and Gerber in 1986. Provide Nanometer-scale analysis to sample surface.
- 10. Cantilever length is 100 - 200 μm. Silicon or Silicon Nitride tips are integrated in the cantilever. Radius of Curvature is 5 – 30nm. SEM Image of a Standard Nano-Probe Cantilever Tip
- 11. A C D B E 1) Line A 2) Line B 3) Line C 4) Line D 5) Lines E & F AFM Force Measurement A, B & C - Approach D, E & F - Retraction Distance of Separation (nm) Tip Deflection (nm) Z X F
- 12. Possible Configuration to Study Bacterial Adhesion by AFM Planar surface (Glass Plate) Bacteria Cantilever with Silicon Nitride Tip
- 13. Escherichia coli (E. coli) K-12, D21 Strain Ong. Y. L. et al., 1999. Lipopolysaccharide (LPS) Outer Membrane Inner Membrane Periplasmic Space Contact Angle Measurements 19.4 ± 3.0 Zeta Potential -28.8 ± 1.7 Surface Properties of E. coli D21
- 14. MATERIALS AND METHODS CELLS + GLUTARALDEHYDE FIXED CELLS POLYETHYLENEIMMINE COATED GLASS POLYETHYLENEIMMINE POLYETHYLENEIMMINE
- 15. Glutaraldehyde Reaction Gluteraldehyde consists of two Aldehyde groups separated by a flexible chain of three methyl groups. In Biological samples, aldehyde group react with free amine groups of proteins. As a result, glutaraldehyde increases cell rigidity.
- 16. AFM Image of Immobilized E. coli
- 17. MODIFIED AFM CANTILEVERS CELLS + GLUTARALDEHYDE FIXED CELLS POLYETHYLENEIMMINE Coated Si 3 N 4 Tips Bacterial Lawn on Si 3 N 4 Tip
- 18. Control Experiments 30nm 70nm
- 19. Results and Discussion Force, F = k x Δ X Spring Constant of Cantilever, k = 0.06nN/nM Δ X = Tip Deflection for the Approach curve.
- 20. Results and Discussion -0.45 ± 0.02 -0.35 ± 0.06 Experiment in PBS+NaCl Experiment in PBS only E. coli bacteria on tip and on glass surface E. coli bacteria on tip and on glass surface Configuration Experiment Force Values in nN
- 21. Approach Retraction Approach Retraction Approach Retraction 35nm 45nm 55nm 12mg/l 18mg/l 24mg/l Experiments with Alum Coagulant
- 22. Results and Discussion Force values for Bacteria – Bacteria Interaction in Different Concentrations of Alum -1.77 ± 0.2 -0.77 ± 0.02 -0.70 ± 0.06 Force in (nN) 24 18 12 Alum Conc. in (mg/l)
- 23. 20nm 25nm 35nm Approach Retraction Approach Retraction Approach Retraction 20mg/l 40mg/l 60mg/l Experiments with Ferric Chloride Coagulant
- 24. Results and Discussion Force values for Bacteria – Bacteria Interaction in Different Concentrations of Ferric Chloride -1.16 ± 0.01 -0.45 ± 0.08 -0.22 ± 0.05 Force in (nN) 60 40 20 FeCl 3 Conc. in (mg/l)
- 25. Conclusions Control Studies (Experiments with NaCl) demonstrate that physiochemical interactions play a dominant role in bacterial adhesion. Alum & Ferric Chloride Coagulants reduce repulsive electrostatic interactions such that attractive forces (primarily van der Waals) become stronger over greater distance of separation. The AFM-methodology makes it possible to optimize coagulation conditions by providing quantitative data (force versus distance of separation curves).
- 26. Interactions between cryptosporidium oocysts in different concentrations of coagulants. Future Experiments No interaction was observed between Cryptosporidium in PBS. Cryptosporidium Cryptosporidium Lawn
- 27. AFM Image of Cryptosporidium oocysts
- 28. Future Work Microbes Microbial Lawn Other Microbial cells commonly found in water 1. Inorganic Particles Microbes 2. Sediment-coated cantilever probing sediment-coated substrate Inorganic Particle Inorganic Particles 3.
- 29. Advisors - Dr. Morteza Abbaszadegan & Dr. Anneta Razatos Funding Agency – National Science Foundation Water Quality Center Faculty Research Associates - Dr. Absar Alum & Dr. Laura Palmer Lab mates – Hodon, Patricia, Prajakta, Rudy, Shahin, Hamed, Anthony, Gideon, Jay and Rong. Parents and Sister Acknowledgments
- 30. Questions ?
- 31. Arrangement for experiments in fluid
- 32. It is a Physiological Buffer Solution. So the working environment (medium) can be varied without causing much stress to the cells. Any bacteria in PBS is in Isotonic Conditions ( Bacteria is not under any stress due to Osmotic pressure conditions) Why conduct experiments in PBS Phosphate Buffer Saline (PBS) :- 136mM NaCl, 2.68mM KCl, 10.1mM Na 2 PO 4 , 1.37mM KH 2 PO 4
- 33. Difference between Gram-negative & Gram-positive bacteria Gram-negative bacteria Gram-positive bacteria Contains both inner as well as outer lipid bilayers & a thin layer of peptidoglycan. Fail to retain violet stain due to thin peptidoglycan layer. Contains a single lipid bilayers, surrounded by thick peptidoglycan layer and polysaccharides including teichoic acid. Retain crystal violet color due to thick peptidoglycan layer.
- 34. Difference between E. coli D21strain & E. coli bacteria LPS structure of E. coli D21 bacteria with no O-antigens. LPS structure of E. coli bacteria with O-antigens. N-acetyl Glucosamine Lipid A Glucose KDO Heptose Galactose
- 35. Origins of Surface Charge Organic surface (proteins) can contain carboxyl (COO - ) & amino (NH 3 + ) groups becomes charged through ionization reactions as follows, As pH of solution increases (i.e., [H + ] decreases), the surface charge becomes increasingly negative. Proteins have a negative charge at a pH above 4. COOH – R – NH 3 + => COO - – R – NH 3 + --------- (1) COO - – R – NH 3 + => COO - – R – NH 2 + ------------- (2)


































![Origins of Surface Charge Organic surface (proteins) can contain carboxyl (COO - ) & amino (NH 3 + ) groups becomes charged through ionization reactions as follows, As pH of solution increases (i.e., [H + ] decreases), the surface charge becomes increasingly negative. Proteins have a negative charge at a pH above 4. COOH – R – NH 3 + => COO - – R – NH 3 + --------- (1) COO - – R – NH 3 + => COO - – R – NH 2 + ------------- (2)](https://image.slidesharecdn.com/thesispresentation-12743357592199-phpapp01/85/Adhesion-Forces-during-Coagulation-as-Evaluated-by-Atomic-Force-Microscopy-35-320.jpg)