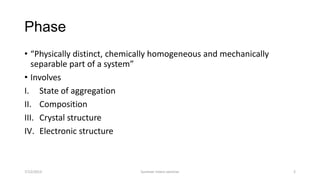
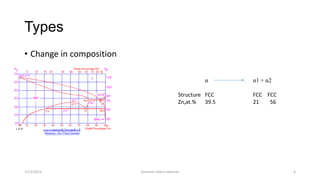
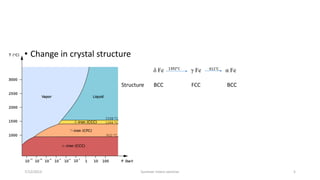
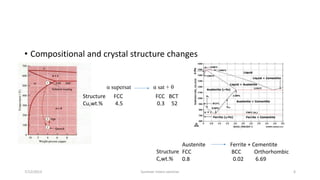
The document discusses different types of solid state phase transformations. It defines a phase as a physically distinct, chemically homogeneous and mechanically separable part of a system, which can involve state of aggregation, composition, crystal structure and electronic structure. It describes atom movements during phase transformations as either long or short range diffusion, or diffusionless changes over a fraction of an interatomic distance. The document then outlines different types of phase transformations, including changes in composition, crystal structure, compositional and crystal structure changes simultaneously, changes in order, and electronic transitions such as the Curie temperature in iron. It concludes by defining homogeneous transformations that occur simultaneously everywhere, versus heterogeneous transformations that involve nucleation and growth.