Micromolecules and Macromolecules
- 2. A biomolecule is any molecule that is present in living organisms, they are divided into macro molecules and micro molecules as follows:- MACROMOLECULES  M > 1000  EXAMPLES 1. Polysaccharides 2. Nucleic acids 3. Proteins MICROMOLECULES  M < 1000  EXAMPLES 1. Amino acids 2. Sugars 3. Nucleotides 4. Lipids
- 3. MACROMOLECULES  There are two kinds of Polysaccharides: 1. HOMOPOLYSACCHARIDES CELLULOSE , STARCH 2. HETEROPOLYSACCHARIDES CHITIN POLYSACCHARIDES
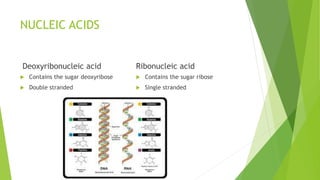
- 4. NUCLEIC ACID  Function- transmits and stores genetic information  Composed of C, H, O, N & P (Phosphorous)  Two types 1. DNA 2. RNA
- 5. NUCLEIC ACIDS Deoxyribonucleic acid  Contains the sugar deoxyribose  Double stranded Ribonucleic acid  Contains the sugar ribose  Single stranded
- 6. PROTIENS  Polymers of amino acids  Organic compound made up of : Carbon Hydrogen Oxygen and Nitrogen  Proteins are essential to living things: Proteins are needed to build & maintain cells, digest food, growth, insulin, antibodies for immunity, transmit heredity, movement.  Examples of Proteins: ◊ Haemoglobin – carries O2 ◊ Actin – muscle contraction ◊ Saliva (Enzyme) – breakdown Carbohydrates. ◊ Lactase (Enzyme) – digest lactose sugar  Four Levels of Protein Structure 1. The primary structure of a protein is its unique sequence of amino acids 2. Secondary structure, found in most proteins, consists of coils and folds in the polypeptide chain 3. Tertiary structure is determined by interactions among various side chains (R groups) 4. Quaternary structure results when a protein consists of multiple polypeptide chains Animation
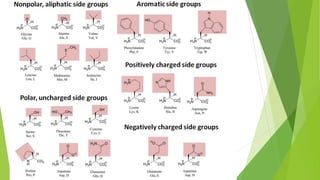
- 8. MICRO MOLECULES  There are 20 different amino acids that are incorporated into proteins.  All amino acids have an Amino Group (NH2), a Carboxyl group (COOH), and an R-Group (unique side chain that distinguishes that amino acid).  Amino acids differ in their properties due to differing side chains, called R groups  The sequence of amino acids determines a protein’s three-dimensional structure. A protein’s structure determines its function. AMINO ACIDS
- 10. SUGARS  Monosaccharides : simplest sugars, which cannot be hydrolysed further into smaller sugars  Composed of 3-7 C atoms 1. Triose (3C) (Glyceraldehyde) 2. Tetrose (4C) (Erythrose) 3. Pentose (5C) (Ribose) 4. Hexose (6C) (Glucose) 5. Heptose (7C) (Sedoheptulose)  Oligosaccharides : when 2/ few monosaccharides are combined by glycosidic bonds.They are named as: 1. Disaccharides (2) : Sucrose 2. Trisaccharides (3) : Arabinose 3. Tetrasaccharides (4) :Stachyose 4. Pentasaccharides (5) : Verbascose
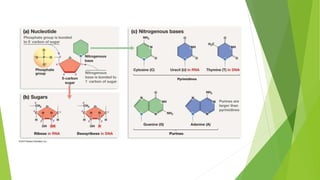
- 12. NUCLEOTIDES  Nucleotides are organic molecules that serve as the monomers, or subunits, of nucleic acids like DNA and RNA.  The building blocks of nucleic acids, nucleotides are composed of a nitrogenous base, a five-carbon sugar (ribose or deoxyribose), and at least one phosphate group.  Nitrogenous base attached to pentose sugar – adenosine, guanosine , thymidine, cytidine & uridine.
- 14. LIPIDS  Fats, oils, waxes, steroids (examples)  Are made mostly of carbon, hydrogen, and oxygen  Are not soluble in water (they are nonpolar)  Hydrogen : oxygen ratio is greater than 2:1  Functions of Lipids 1. Used to store energy 2. Important part of biological membranes  There are two type: 1. Saturated Lipids : Solid fats, animals 2. Unsaturated Lipids: Oils, plants  Steroids are lipids characterized by a carbon skeleton consisting of four fused rings . Cholesterol, an important steroid, is a component in animal cell membranes . Although cholesterol is essential in animals, high levels in the blood may contribute to cardiovascular disease.