Medtec2013 v1jn
1 like312 views
This document discusses balancing regulations with engineering innovation when designing medical products. It addresses key aspects of regulation including timelines, elements, enforcement, and common pitfalls. Engineering fields like mechanical, manufacturing, and software engineering are discussed. The presentation emphasizes finding a balance between meeting regulations and advancing engineering through commitment and considering equipment, processes, quality, and documentation. An example of a CNC machine with integrated quality control is provided. References for further information on FDA regulations and guidance documents are also included to spark discussion.
1 of 10
Download to read offline






Ad
Recommended
Knowledge Managemet- Poster
Knowledge Managemet- PosterSanket S. Lingayat
Ěý
The document proposes developing a software tool to track, model, and rationalize engineering workflows. The tool would collect and analyze metadata about work products to identify long feedback loops and inconsistencies caused by issues earlier in the process. It would help detect deviations and mismatches between work products on specific programs. The goal is to enhance configuration management and avoid unnecessary rework through in-process testing and tracking workflows.McAllister Consulting - Good documentation practices
McAllister Consulting - Good documentation practicesDoug Bryson
Ěý
This document provides an overview of good documentation practices (GDP) for complying with FDA regulations. It discusses the importance of document and records control procedures according to ISO 9001 and 21 CFR 820 standards. Key elements that are highlighted include using indelible ink, not using white out, filling in all record fields, signing and dating entries, numbering pages for clarity, retaining records according to procedures, and ensuring records are retrievable. Electronic records and documents must also follow the same control principles of passwords, security, backups, and storing records in secure locations. The presentation recommends reviewing current practices, training employees, implementing GDP immediately, communicating any issues, and being prepared for future audits.Predictive maintenance
Predictive maintenanceAdvisian
Ěý
This document discusses why it has been difficult to implement predictive maintenance approaches. It describes three situations for one of the client's facilities where predictive maintenance could help reduce costs and personnel: 1) A new facility that could reduce on-site staff, 2) An unmanned installation that wants to reduce visit frequency, and 3) Platforms that want to visit just once a year. The document then provides an in-depth discussion on how to better implement predictive maintenance by addressing organizational and cultural barriers.Datasheet ke-operating-room
Datasheet ke-operating-roomHealth Care DataWorks
Ěý
This document describes an operating room dashboard that provides metrics and key performance indicators to assess operating room efficiency. The dashboard allows users to view overall performance trends over time as well as drill down into specific details by department, surgeon, or operating room. It addresses questions about on-time starts and finishes, room utilization, throughput times, and compliance with safety and infection control protocols. The dashboard is part of a business intelligence software suite that helps healthcare organizations improve patient care, costs, and operations through data-driven insights.Complying with 21 CFR Part 11 - Understanding the role of predicate rule
Complying with 21 CFR Part 11 - Understanding the role of predicate ruleJasmin NUHIC
Ěý
The document provides an overview of 21 CFR Part 11, emphasizing the regulation's implications for electronic records and signatures, and the consequences of non-compliance. It outlines key definitions, subparts detailing general provisions for electronic records and signatures, and the necessary controls to ensure authenticity, integrity, and confidentiality. The presentation concludes with a discussion on audit implications, training, and options for addressing non-compliance.REFERENCES 11.2015
REFERENCES 11.2015SOCOTEC BELGIUM SPRL / BVBA
Ěý
This document provides descriptions of various construction, renovation, and facility management projects that SOCOTEC Belgium has completed. It lists services provided such as design reviews, site inspections, safety and technical system evaluations, and energy audits. Projects include the construction of the NATO headquarters, hospitals, a pedestrian bridge, shopping centers, office buildings, and facilities for the European Parliament and European Space Agency.Netherlands
NetherlandsPilarJimenezGomez
Ěý
Netherlands is known for its tulips, windmills, and canals. The capital is Amsterdam. Some of the top attractions include museums, flowers, windmills, and bike tours. The climate is maritime and temperate with cold winters and mild summers. Common Dutch foods include cheeses, breads, and herbs. Primary education is completed around age twelve but students remain in additional schooling.´ł´Ç˛ą±çłÜòÔprofe-oscar
Ěý
Cuando un meteorito impacta, crea una onda de choque que funde el meteorito y se expande. Luego, el polvo eyectado se dispersa mientras el meteorito rebota. Los meteoritos pueden estar compuestos de gases congelados, agua o ser rocosos o metálicos de hierro. Los telescopios y satélites se usan para descubrir y observar meteoritos u otros cuerpos celestes.Ejercicio Word 15Niieves_07
Ěý
El documento analiza la volatilidad del comportamiento de las petroleras en bolsa en las Ăşltimas semanas desde los ataques terroristas del 11 de septiembre. La primera caĂda fuerte en el precio del crudo se debiĂł a tres factores: el miedo a una reducciĂłn de la demanda de combustible para aviones a corto plazo, la reducciĂłn de la demanda general de petrĂłleo debido a una economĂa más dĂ©bil y el riesgo de recesiĂłn, y el bajo cumplimiento de las cuotas de producciĂłn por parte de la OPEPJani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...
Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...Kelan tutkimus / Research at Kela
Ěý
Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet - esimerkkejä. Esitys seminaarissa "Kelan etuudet sotessa – onko monikanavarahoitus pian historiaa?" 1.12.2015. Kelan päätalo, Helsinki.Historia de RomaJaime Molina
Ěý
Este documento presenta una introducciĂłn a la historia de la Roma antigua, desde su fundaciĂłn mĂtica y histĂłrica hasta el Imperio y la divisiĂłn de Bizancio. Cubre temas como la monarquĂa, repĂşblica, imperio, clases sociales, instituciones polĂticas, religiĂłn, guerras pĂşnicas y derecho romano. El documento incluye diapositivas con informaciĂłn sobre cada uno de estos aspectos de la sociedad y cultura romana antigua.LDO07 Miroslav OpatrnĂ˝
LDO07 Miroslav OpatrnĂ˝Asociace.BIZ - Asociace dodavatelĹŻ internetovĂ˝ch Ĺ™ešenĂ, o.s.
Ěý
Prezentace Miroslava OpatrnĂ©ho z konference La Degustation Online vÄ›novanĂ© vÄ›rnostnĂm programĹŻm.Solsticio2quimiprofe
Ěý
El documento describe los solsticios de verano e invierno. En los dĂas de solsticio, la duraciĂłn del dĂa y la altitud del Sol al mediodĂa son máximas o mĂnimas comparadas con otros dĂas del año. En muchas culturas antiguas se celebraban festivales en los solsticios. Los solsticios ocurren dos veces al año cuando el Sol alcanza su punto más alto o bajo en el cielo sobre los trĂłpicos de Cáncer y Capricornio.Ia%20 %20 conceptos%20e%20historia%20version%20ms%20office%202003barcelu
Ěý
La I.A. ha pasado por varias etapas históricas, incluyendo su génesis en las décadas de 1940 y 1950, un entusiasmo inicial en las décadas de 1950 y 1960, un enfoque en sistemas basados en el conocimiento en la década de 1970, y su conversión en una industria y ciencia en las décadas posteriores.Convergence
ConvergenceLaura DeLea
Ěý
RCM sees convergence, the combining and integrating of IT, engineering, and validation skills, as driving change in the bioeconomy. As technologies converge, organizations that do not embrace convergence across skills will fall behind. Regulatory pressures are forcing greater integration of functions. Convergence demands broader skillsets to validate infrastructures and ensure compliance. RCM's combination of IT, validation, and engineering expertise helps clients in regulated industries address the challenges of compliance in a converging landscape.MayorTek Technical Services - Overview
MayorTek Technical Services - OverviewMarco Mayor
Ěý
The document provides an overview of technical services offered by MayorTek including product development, engineering design, quality management, project management, and various specialized services. Key services described include mechanical, electrical, and software engineering; industrial design; systems architecture; testing; regulatory compliance; value analysis; and approaches to optimize design such as design for manufacturing and quality.Quality Re Pres Ebert Rudorfer Med Conf2011 V5
Quality Re Pres Ebert Rudorfer Med Conf2011 V5Arnold Rudorfer
Ěý
This document discusses quality requirements engineering for medical systems. It provides an overview of quality requirements engineering challenges for medical device projects. It then discusses Siemens and Vector Consulting, the business environment for medical devices, and examples of applying quality requirements engineering for security. The document outlines some case studies and results, concluding that quality requirements engineering can improve system reliability and availability while reducing engineering effort.Quality Re Pres Ebert Rudorfer Med Conf2011 V4
Quality Re Pres Ebert Rudorfer Med Conf2011 V4Arnold Rudorfer
Ěý
This document summarizes a presentation on quality requirements engineering for medical systems. It discusses the challenges of developing safety and security critical medical devices. It provides an overview of quality requirements engineering and examples of how it was applied to a large medical device project at Siemens Healthcare involving thousands of requirements and hundreds of developers. The presentation outlines issues addressed through solutions like a feature model, forced ranking, architecture mapping, and a quality tree. It discusses positive results like improved reliability and reduced review efforts.Codex validation Group presentation
Codex validation Group presentationWalter Acevedo
Ěý
Codex Validation Group is an engineering staffing and validation services company founded in Puerto Rico by professionals with over 25 years of combined experience in the pharmaceutical industry. The company provides validation, engineering, IT, and regulatory compliance services with a focus on computer and automation system validation. Its team of experts have experience across the product development lifecycle from process engineering to packaging and equipment qualification. The company aims to become a leader in technical services and regulatory compliance solutions for manufacturing clients.Rapid Product Innovation
Rapid Product InnovationARC Advisory Group
Ěý
1) Today's business environment requires rapid product and process innovation to address global competition, changing customer demands, and increasing costs and regulatory pressures.
2) Introducing new products or improving manufacturing processes often requires modifying production systems, but many issues are not discovered until late in the design/implementation process, introducing delays and costs.
3) Current digital tools for designing, modeling, and simulating manufacturing systems have limitations and often do not integrate well, hindering collaboration between different engineering disciplines involved in production system development.CRI Company Overview 2012
CRI Company Overview 2012jmeinertCRI
Ěý
Catheter Research, Inc. (CRI) is a medical device contract manufacturer and developer specializing in catheters and tubing. It has over 130 employees and was established in 1987. CRI provides cost-effective product development and quality OEM manufacturing services. It has a fully integrated quality system and earned ISO 13485 certification. CRI focuses on lean manufacturing, customer service, and community involvement.Quality systems v3
Quality systems v3Ravi Pamnani
Ěý
The document provides an overview of quality systems for medical device manufacturers. It discusses the components required by the FDA's Quality System Regulation, including management responsibility, design controls, document controls, purchasing controls, production and process controls, and acceptance activities. It emphasizes that quality systems help ensure consistent compliance with requirements and specifications to improve safety and efficacy.BQ-Engg Design Services 2012
BQ-Engg Design Services 2012NEERAJ SRIVASTAVA
Ěý
BQT is an engineering services company with offices in India, the US, and the UK. They provide CAD, CAM, CAE, and PDM engineering services including 3D modeling, simulation, prototyping, and product design and development. Their team of qualified engineers help reduce costs and time-to-market while maintaining quality, flexibility, and intellectual property security for clients.MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURING
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURINGMessung Workplace Technology
Ěý
Messung is a leading industrial technology expert in India focused on providing advanced automation and control solutions, particularly for the pharmaceutical industry. The company offers a comprehensive range of products and services, including process automation, energy management systems, and workplace technology, all tailored to meet strict industry standards and regulatory compliance. With decades of experience, Messung aims to enhance operational efficiency and product quality for its clients while upholding environmental responsibility.Caso Lixis HEC
Caso Lixis HECenendeavor
Ěý
The document outlines Lixis's strategy to enter the European pharmaceutical market, focusing on their value proposition of quality control equipment at competitive prices and enhanced before-and-after sales services. It emphasizes the importance of addressing industry needs, forming strategic partnerships, and providing installations and validation as differentiating factors. Additionally, it highlights the necessity of a robust business model to build customer trust and ensure sustainable market presence.Crisp present-oct-2009
Crisp present-oct-2009kunjesh2011
Ěý
This document describes a premier technical training institute called CRISP that provides high-quality training, consultancy, and job services in advanced technology areas. It offers competency-based and modular programs to industry personnel, job seekers, government departments and technical teachers. Its facilities and programs cover areas like industrial automation, manufacturing technology, information technology, behavioral science, handicraft development and vocational training. The goal is to enhance industry and workforce competitiveness through skills enhancement and improved employment prospects.More Related Content
Viewers also liked (10)
´ł´Ç˛ą±çłÜòÔprofe-oscar
Ěý
Cuando un meteorito impacta, crea una onda de choque que funde el meteorito y se expande. Luego, el polvo eyectado se dispersa mientras el meteorito rebota. Los meteoritos pueden estar compuestos de gases congelados, agua o ser rocosos o metálicos de hierro. Los telescopios y satélites se usan para descubrir y observar meteoritos u otros cuerpos celestes.Ejercicio Word 15Niieves_07
Ěý
El documento analiza la volatilidad del comportamiento de las petroleras en bolsa en las Ăşltimas semanas desde los ataques terroristas del 11 de septiembre. La primera caĂda fuerte en el precio del crudo se debiĂł a tres factores: el miedo a una reducciĂłn de la demanda de combustible para aviones a corto plazo, la reducciĂłn de la demanda general de petrĂłleo debido a una economĂa más dĂ©bil y el riesgo de recesiĂłn, y el bajo cumplimiento de las cuotas de producciĂłn por parte de la OPEPJani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...
Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...Kelan tutkimus / Research at Kela
Ěý
Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet - esimerkkejä. Esitys seminaarissa "Kelan etuudet sotessa – onko monikanavarahoitus pian historiaa?" 1.12.2015. Kelan päätalo, Helsinki.Historia de RomaJaime Molina
Ěý
Este documento presenta una introducciĂłn a la historia de la Roma antigua, desde su fundaciĂłn mĂtica y histĂłrica hasta el Imperio y la divisiĂłn de Bizancio. Cubre temas como la monarquĂa, repĂşblica, imperio, clases sociales, instituciones polĂticas, religiĂłn, guerras pĂşnicas y derecho romano. El documento incluye diapositivas con informaciĂłn sobre cada uno de estos aspectos de la sociedad y cultura romana antigua.LDO07 Miroslav OpatrnĂ˝
LDO07 Miroslav OpatrnĂ˝Asociace.BIZ - Asociace dodavatelĹŻ internetovĂ˝ch Ĺ™ešenĂ, o.s.
Ěý
Prezentace Miroslava OpatrnĂ©ho z konference La Degustation Online vÄ›novanĂ© vÄ›rnostnĂm programĹŻm.Solsticio2quimiprofe
Ěý
El documento describe los solsticios de verano e invierno. En los dĂas de solsticio, la duraciĂłn del dĂa y la altitud del Sol al mediodĂa son máximas o mĂnimas comparadas con otros dĂas del año. En muchas culturas antiguas se celebraban festivales en los solsticios. Los solsticios ocurren dos veces al año cuando el Sol alcanza su punto más alto o bajo en el cielo sobre los trĂłpicos de Cáncer y Capricornio.Ia%20 %20 conceptos%20e%20historia%20version%20ms%20office%202003barcelu
Ěý
La I.A. ha pasado por varias etapas históricas, incluyendo su génesis en las décadas de 1940 y 1950, un entusiasmo inicial en las décadas de 1950 y 1960, un enfoque en sistemas basados en el conocimiento en la década de 1970, y su conversión en una industria y ciencia en las décadas posteriores.Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...
Jani Miettinen: Oulun SOTEa kuvaava rekisteriaineisto ja sen mahdollisuudet -...Kelan tutkimus / Research at Kela
Ěý
Similar to Medtec2013 v1jn (20)
Convergence
ConvergenceLaura DeLea
Ěý
RCM sees convergence, the combining and integrating of IT, engineering, and validation skills, as driving change in the bioeconomy. As technologies converge, organizations that do not embrace convergence across skills will fall behind. Regulatory pressures are forcing greater integration of functions. Convergence demands broader skillsets to validate infrastructures and ensure compliance. RCM's combination of IT, validation, and engineering expertise helps clients in regulated industries address the challenges of compliance in a converging landscape.MayorTek Technical Services - Overview
MayorTek Technical Services - OverviewMarco Mayor
Ěý
The document provides an overview of technical services offered by MayorTek including product development, engineering design, quality management, project management, and various specialized services. Key services described include mechanical, electrical, and software engineering; industrial design; systems architecture; testing; regulatory compliance; value analysis; and approaches to optimize design such as design for manufacturing and quality.Quality Re Pres Ebert Rudorfer Med Conf2011 V5
Quality Re Pres Ebert Rudorfer Med Conf2011 V5Arnold Rudorfer
Ěý
This document discusses quality requirements engineering for medical systems. It provides an overview of quality requirements engineering challenges for medical device projects. It then discusses Siemens and Vector Consulting, the business environment for medical devices, and examples of applying quality requirements engineering for security. The document outlines some case studies and results, concluding that quality requirements engineering can improve system reliability and availability while reducing engineering effort.Quality Re Pres Ebert Rudorfer Med Conf2011 V4
Quality Re Pres Ebert Rudorfer Med Conf2011 V4Arnold Rudorfer
Ěý
This document summarizes a presentation on quality requirements engineering for medical systems. It discusses the challenges of developing safety and security critical medical devices. It provides an overview of quality requirements engineering and examples of how it was applied to a large medical device project at Siemens Healthcare involving thousands of requirements and hundreds of developers. The presentation outlines issues addressed through solutions like a feature model, forced ranking, architecture mapping, and a quality tree. It discusses positive results like improved reliability and reduced review efforts.Codex validation Group presentation
Codex validation Group presentationWalter Acevedo
Ěý
Codex Validation Group is an engineering staffing and validation services company founded in Puerto Rico by professionals with over 25 years of combined experience in the pharmaceutical industry. The company provides validation, engineering, IT, and regulatory compliance services with a focus on computer and automation system validation. Its team of experts have experience across the product development lifecycle from process engineering to packaging and equipment qualification. The company aims to become a leader in technical services and regulatory compliance solutions for manufacturing clients.Rapid Product Innovation
Rapid Product InnovationARC Advisory Group
Ěý
1) Today's business environment requires rapid product and process innovation to address global competition, changing customer demands, and increasing costs and regulatory pressures.
2) Introducing new products or improving manufacturing processes often requires modifying production systems, but many issues are not discovered until late in the design/implementation process, introducing delays and costs.
3) Current digital tools for designing, modeling, and simulating manufacturing systems have limitations and often do not integrate well, hindering collaboration between different engineering disciplines involved in production system development.CRI Company Overview 2012
CRI Company Overview 2012jmeinertCRI
Ěý
Catheter Research, Inc. (CRI) is a medical device contract manufacturer and developer specializing in catheters and tubing. It has over 130 employees and was established in 1987. CRI provides cost-effective product development and quality OEM manufacturing services. It has a fully integrated quality system and earned ISO 13485 certification. CRI focuses on lean manufacturing, customer service, and community involvement.Quality systems v3
Quality systems v3Ravi Pamnani
Ěý
The document provides an overview of quality systems for medical device manufacturers. It discusses the components required by the FDA's Quality System Regulation, including management responsibility, design controls, document controls, purchasing controls, production and process controls, and acceptance activities. It emphasizes that quality systems help ensure consistent compliance with requirements and specifications to improve safety and efficacy.BQ-Engg Design Services 2012
BQ-Engg Design Services 2012NEERAJ SRIVASTAVA
Ěý
BQT is an engineering services company with offices in India, the US, and the UK. They provide CAD, CAM, CAE, and PDM engineering services including 3D modeling, simulation, prototyping, and product design and development. Their team of qualified engineers help reduce costs and time-to-market while maintaining quality, flexibility, and intellectual property security for clients.MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURING
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURINGMessung Workplace Technology
Ěý
Messung is a leading industrial technology expert in India focused on providing advanced automation and control solutions, particularly for the pharmaceutical industry. The company offers a comprehensive range of products and services, including process automation, energy management systems, and workplace technology, all tailored to meet strict industry standards and regulatory compliance. With decades of experience, Messung aims to enhance operational efficiency and product quality for its clients while upholding environmental responsibility.Caso Lixis HEC
Caso Lixis HECenendeavor
Ěý
The document outlines Lixis's strategy to enter the European pharmaceutical market, focusing on their value proposition of quality control equipment at competitive prices and enhanced before-and-after sales services. It emphasizes the importance of addressing industry needs, forming strategic partnerships, and providing installations and validation as differentiating factors. Additionally, it highlights the necessity of a robust business model to build customer trust and ensure sustainable market presence.Crisp present-oct-2009
Crisp present-oct-2009kunjesh2011
Ěý
This document describes a premier technical training institute called CRISP that provides high-quality training, consultancy, and job services in advanced technology areas. It offers competency-based and modular programs to industry personnel, job seekers, government departments and technical teachers. Its facilities and programs cover areas like industrial automation, manufacturing technology, information technology, behavioral science, handicraft development and vocational training. The goal is to enhance industry and workforce competitiveness through skills enhancement and improved employment prospects.Tridiagona Solutions Corporate Presentation
Tridiagona Solutions Corporate Presentationabhijeetsd
Ěý
Tridiagonal Solutions provides customized engineering solutions and develops products by harnessing computational modeling. It focuses on developing solutions from initial concepts to commercial practices through various stages including CFD modeling, process engineering, pilot plants, and product development. Tridiagonal has expertise in industries like oil/gas, chemicals, pharmaceuticals, power and manufacturing.DSI Concept Through Commission
DSI Concept Through Commissiondbirchmeier
Ěý
Design Systems, Inc. is a multi-disciplinary engineering firm specializing in manufacturing process design and integration. They provide turnkey systems integration and engineering services worldwide from concept to commissioning. Their services include manufacturing process engineering, simulation engineering, electrical and controls engineering, and more.MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURING
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURINGMessung Workplace Technology
Ěý
Messung specializes in process automation solutions tailored for the pharmaceutical industry, emphasizing quality control and regulatory compliance through integrated systems for machine automation, wastewater treatment, HVAC, energy management, and workplace ergonomics. With decades of experience, they provide customized technology solutions that improve production efficiency while ensuring safety and adherence to strict guidelines. Their extensive product portfolio and partnerships enhance capabilities in diverse areas such as high-speed packaging and energy conservation in pharma manufacturing.Aras Partner Solution by Minerva
Aras Partner Solution by MinervaAras
Ěý
The document introduces the Minerva Product Lifecycle Management (PLM) solution for the electronics, high-tech, and medical device industries. It highlights key capabilities like change management, collaboration with external partners, quality management features, and regulatory compliance tools. The solution is demonstrated through the example of its implementation at a Swedish electronics control systems company called CPAC AB. Contact information is provided for Leon Lauritsen of Minerva to answer any additional questions.PLM - ERP integration
PLM - ERP integrationHenri Moufettal
Ěý
The document discusses the integration of Product Lifecycle Management (PLM) and Enterprise Resource Planning (ERP) systems to enhance collaboration in value-added activities during new product implementation. It outlines the interactions between engineering and manufacturing, emphasizing the role of 3D modeling and the management of engineering and manufacturing bills of materials. Successful integration offers strategic advantages, including improved cost management and streamlined processes across product development and operational phases.Auditing of Quality Asurrance & Engg. Dept..pptx
Auditing of Quality Asurrance & Engg. Dept..pptxSiddhiShrigiriwar
Ěý
This log provides the information about the Audit of Quality and Engineering Department. It consists of Critical Systems HVAC, WFI, Effluent Treatment Plant(ETP) .
Audit system and validation.Dsi Concept Through Commission
Dsi Concept Through Commissionpmunzen
Ěý
Design Systems, Inc. is a multi-disciplined engineering firm specializing in manufacturing process design and integration. They provide turnkey systems integration and engineering services worldwide from concept to commissioning. They have over 200 engineers and technicians with expertise in areas such as industrial engineering, simulation, process engineering, electrical and controls, and mechanical and facilities engineering. Their goal is to help clients maximize production capacity through efficient manufacturing design.Technology and design
Technology and designArbnor Hoxhaj
Ěý
Technology and design (T.D.) involves the study, design, development, application, and management of computer and non-computer technologies to communicate design intent, facilitate product operation and maintenance, and improve overall product realization. Design is the creation of a plan for an object's construction, while technology refers to tools used by humans to control their environments. Computer-aided design (CAD) uses computers to assist in the creation, modification, analysis, or optimization of a design. CAD and computer-aided engineering (CAE) are important tools that are widely used in industries such as automotive, shipbuilding, and aerospace. Product lifecycle management (PLM) integrates people, data, processes and systems toMESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURING
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURINGMessung Workplace Technology
Ěý
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURING
MESSUNG’S EXPERTISE IN ENSURING HEALTHY OPERATIONS FOR PHARMA MANUFACTURINGMessung Workplace Technology
Ěý
Ad
More from Jasmin NUHIC (6)
Stuck at Home: What to do about your professional development
Stuck at Home: What to do about your professional developmentJasmin NUHIC
Ěý
The document outlines best practices for participating in webinars and emphasizes the importance of professional development while at home. It highlights a nine-step process for creating an executable individual development plan (eidp) and suggests activities like self-assessments and networking for personal growth. Resources and references are provided to support ongoing professional development.Global Project Manager: Beyond the Title
Global Project Manager: Beyond the TitleJasmin NUHIC
Ěý
The document discusses the challenges and solutions associated with leading global projects, emphasizing the importance of emotional intelligence, cultural respect, and diverse experiences. It suggests practical tools, websites, and educational resources to enhance global project management skills. Additionally, it highlights the significance of co-mentoring and co-coaching for professional development in this field.Canadian policy for combination (drug / device) products
Canadian policy for combination (drug / device) productsJasmin NUHIC
Ěý
The Health Canada policy for drug/medical device combination products aims to streamline submission processes by allowing such products to be regulated under either food and drug regulations or medical devices regulations, based on their principal mechanism of action. This approach is intended to reduce regulatory burdens for sponsors and align Canadian regulations with those of the U.S. and European Union. The policy is effective as of March 1, 2006, and provides interim guidelines until permanent regulatory amendments can be enacted.21 CFR Part 4 - CGMP for Combination Products
21 CFR Part 4 - CGMP for Combination ProductsJasmin NUHIC
Ěý
The document outlines the final rule for 21 CFR Part 4, effective July 22, 2013, which clarifies current good manufacturing practice (CGMP) requirements for combination products combining drugs, devices, and biological products. It aims to ensure consistent application and enforcement of these requirements to promote public health while providing a transparent regulatory framework. The rule includes detailed sections on scope, definitions, applicable CGMPs, and compliance for single-entity and co-packaged combination products.ISO 14001 Environmental Management
ISO 14001 Environmental ManagementJasmin NUHIC
Ěý
The document discusses ISO 14000 and the implementation of an environmental management system (EMS) based on ISO 14001 standards. It details the requirements for establishing an EMS, the benefits of compliance, and tips for effective implementation, including planning, execution, monitoring, and management review. Key benefits of ISO 14001 include improved environmental performance, cost reductions, and enhanced reputation in the global marketplace.Principles and Practices of Traceability and Calibration
Principles and Practices of Traceability and CalibrationJasmin NUHIC
Ěý
The document provides an overview of measurement units, calibration standards, and traceability principles, aimed at educating readers on the essential concepts of measurement practices. It covers base and derived SI units, types of measurement standards, common measurement techniques, and the importance of traceability in ensuring measurement accuracy. Additionally, it outlines procedures for substituting calibration standards when necessary.Ad
Medtec2013 v1jn
- 1. Balancing and conforming with existing standards whilst designing innovative products BALANCE Regulation and Engineering Jasmin NUHIC Senior Manager, Manufacturing Engineering Medtronic, Inc February, 2013
- 2. Changing Minds MEDTEC ¦ Stuttgart, 2013 2
- 3. Topics • Regulation • Engineering • Balance • Example • References • Discussion MEDTEC ¦ Stuttgart, 2013 3
- 4. Regulation • Timeline • Elements – General – Scope – Electronic records – Electronic signatures – Audit trails • Enforcement MEDTEC ¦ Stuttgart, 2013 4
- 5. Regulation (cont`d) • Target areas • Common audit findings • Common pitfalls "All Part 11 systems must be validated; • Benefits of being yet not all validated systems must be Part 11 compliant" compliant • Training MS Word «Track Changes» is 21 CFR Part 11 compliant? MEDTEC ¦ Stuttgart, 2013 5
- 6. Engineering • Mechanical (equipment) eng. • Manufacturing eng. • Industrial eng. • Software eng. • Process eng. • Quality eng. • R&D eng. • ...... eng. MEDTEC ¦ Stuttgart, 2013 6
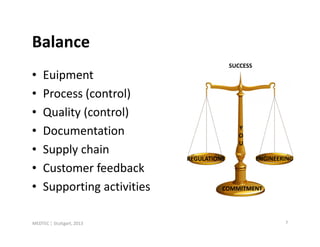
- 7. Balance SUCCESS • Euipment • Process (control) • Quality (control) Y • Documentation O U • Supply chain REGULATIONS ENGINEERING • Customer feedback • Supporting activities COMMITMENT MEDTEC ¦ Stuttgart, 2013 7
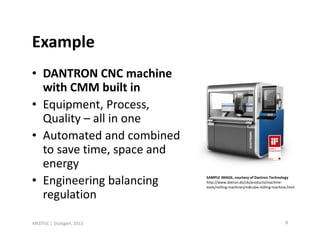
- 8. Example • DANTRON CNC machine with CMM built in • Equipment, Process, Quality – all in one • Automated and combined to save time, space and energy • Engineering balancing SAMPLE IMAGE, courtesy of Dantron Technology http://www.datron.de/uk/products/machine- tools/milling-machines/m8cube-milling-machine.html regulation MEDTEC ¦ Stuttgart, 2013 8
- 9. References • FDA Website – www.fda.gov/ora/compliance_ref/part11 • International Society for Pharmaceutical Engineering (ISPE) – GAMP5, A Risk-Based Approach to Compliant GxP Computerized Systems • John Murray – Software and Compliance Specialist with FDA (Part 11 Subject Matter Expert and advisor) MEDTEC ¦ Stuttgart, 2013 9
- 10. Discussion THANK YOU! MEDTEC ¦ Stuttgart, 2013 10
