Lignin Depolymerization and Conversion
- 1. Lis Nimani 2011 Wisconsin Bioenergy Summit October 6th, 2011 University of Wisconsin - Madison Biological Systems Engineering
- 2. ’üĮ Introduction ’üĮ Lignin Isolation ’üĮ Depolymerization and Conversion of Lignin ŌŚ” Pyrolysis (thermolysis) ŌŚ” Gasification ŌŚ” Hydrogenolysis ŌŚ” Chemical Oxidation ŌŚ” Catalysis ŌŚ” Hydrolysis under Supercritical Conditions
- 3. ’üĮ Renewable Energy sources are needed to replace Fossil Fuels ŌŚ” Depletion of Traditional Fossil Fuels ŌŚ” Global warming due to GHGŌĆÖs ŌŚ” National Security ’üĮ Biomass as an Energy source ŌŚ” Corn/Sugarcane (1st Generation) ŌŚ” Lignocellulosic Biomass (2nd Generation) ŌŚ” Algae (3rd Generation) ’üĮ Lignocellulosic Biomass ŌŚ” Promising Biomass Source ŌŚ” Cellulose, Hemicellulose and Lignin
- 4. ’üĮ Biorefineries convert biomass into useful energy and value added products. ŌŚ” Cellulose and Hemicelluloses are generally converted to ethanol. ŌŚ” Lignin is normally combusted for Heat. ’é¢ Low value ’é¢ Underutilized ’üĮ Conversion of Lignin into Value Added Products. ŌŚ” 15-30 % of biomass is Lignin ŌŚ” Higher Energy Content than Cellulose ’é¢ 9000-11000 Btu/lb vs. 7300-7500 Btu/lb ŌŚ” Challenge: Lignin is a very complex molecule. It is difficult to decompose and generates very high amounts of solid residue.
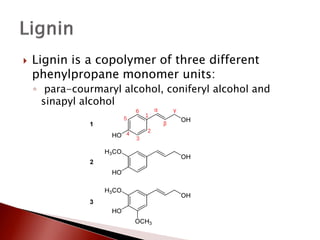
- 5. ’üĮ Lignin is a copolymer of three different phenylpropane monomer units: ŌŚ” para-courmaryl alcohol, coniferyl alcohol and sinapyl alcohol
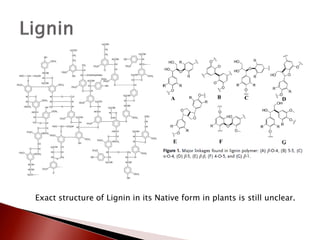
- 6. ’üĮ Peroxidase and laccase enzymes in the plant cell walls cause the dehydrogenation of phenolic OH groups and generate free radicals from these lignin precursors. ’üĮ Polymerization of the lignin monomers starts with the coupling of two radicals forming a dimer ŌŚ” ╬Æ-O-4 aryl ether bonds are the most frequent coupling linkage formed ’üĮ Polymerization progresses with further coupling of monomeric radicals with dimers, trimers and oligomers resulting in complex branched polymer.
- 7. Exact structure of Lignin in its Native form in plants is still unclear.
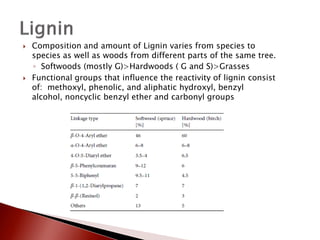
- 8. ’üĮ Composition and amount of Lignin varies from species to species as well as woods from different parts of the same tree. ŌŚ” Softwoods (mostly G)>Hardwoods ( G and S)>Grasses ’üĮ Functional groups that influence the reactivity of lignin consist of: methoxyl, phenolic, and aliphatic hydroxyl, benzyl alcohol, noncyclic benzyl ether and carbonyl groups
- 9. ’üĮ Mechanical vs. Chemical Processes ŌŚ” Lignin can be isolated in a variety of methods ’üĮ Methods can be grouped into two Major Pathways ŌŚ” Cellulose and Hemicelluloses are removed by solubilization, leaving lignin as insoluble residue. ’é¢ Ex. Lignin available as by-product from a lignocellulosic ethanol fuel biorefinery ŌŚ” Methods involving dissolution and removal of lignin, leaving cellulose and hemicelluloses as insoluble residues, followed by the recovery of lignin from the solution. ’é¢ Ex. Kraft and Sulfite Lignin ’üĮ Other Types of Extracted Lignin: ŌŚ” Organosolv (more pure and unaltered lignin due to milder conditions), Milled Wood Lignin (Bjorkman Process), Steam Explosion Lignin, Cellulolytic Enzyme Lignin, Klason Lignin, Acid Hydrolysis Lignin, Soda Lime Lignin, etc. ’üĮ Isolation Method has an influential role in determining the nature and structure of lignin.
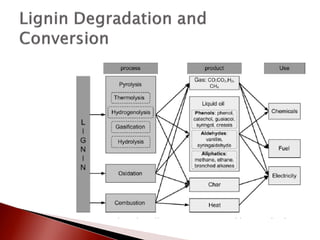
- 10. ’üĮ Degradation and conversion of lignin occurs through thermochemical treatments. ŌŚ” Includes thermal treatment of lignin in the presence or absence of solvents, chemical additives and catalysts. ’üĮ Pyrolysis: Thermal treatment of biomass in the absence of oxygen, with or without catalyst. ’üĮ Gasification: converts lignin to gasses. Major products are H2,CO,CO2 and CH4. ’üĮ Hydrogenolysis: involves thermal treatment in the presence of hydrogen so that the cleavage of bongs is assisted by the addition of hydrogen. Used at lower temperatures favoring higher yields of liquid. ’üĮ Oxidation: Thermal treatment in the presence of oxygen. Lignin to aldehydes. ’üĮ Comubustion: Burning the Lignin to create heat and or electricity. ’üĮ Note: Yields and composition of degradation products vary based on the process and the conditions applied. Also, the nature of the lignin and its various composition and functional groups have significant effects on the lignin conversion and product yields.
- 12. ’üĮ Pyrolysis is the most studied method for the conversion of biomass to lower-molecular weight liquid or gaseous products. ’üĮ Def: Heating an organic substance in the absence of air so that the molecular structure is broken down into smaller units while the limited oxygen available for the reaction ensures that there is no further combustion to Carbon Dioxide. ’üĮ Pyrolysis of lignin is complex and is affected by several factors: feedstock type, heating rate, reaction temperature, additives, etc.
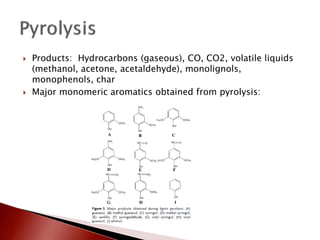
- 13. ’üĮ Products: Hydrocarbons (gaseous), CO, CO2, volatile liquids (methanol, acetone, acetaldehyde), monolignols, monophenols, char ’üĮ Major monomeric aromatics obtained from pyrolysis:
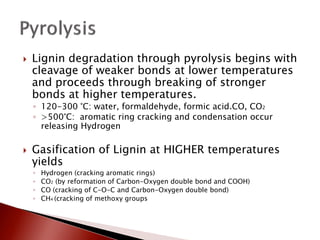
- 14. ’üĮ Lignin degradation through pyrolysis begins with cleavage of weaker bonds at lower temperatures and proceeds through breaking of stronger bonds at higher temperatures. ŌŚ” 120-300 ┬░C: water, formaldehyde, formic acid.CO, CO2 ŌŚ” >500┬░C: aromatic ring cracking and condensation occur releasing Hydrogen ’üĮ Gasification of Lignin at HIGHER temperatures yields ŌŚ” Hydrogen (cracking aromatic rings) ŌŚ” CO2 (by reformation of Carbon-Oxygen double bond and COOH) ŌŚ” CO (cracking of C-O-C and Carbon-Oxygen double bond) ŌŚ” CH4 (cracking of methoxy groups
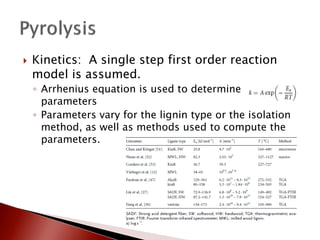
- 15. ’üĮ Kinetics: A single step first order reaction model is assumed. ŌŚ” Arrhenius equation is used to determine rate parameters ŌŚ” Parameters vary for the lignin type or the isolation method, as well as methods used to compute the parameters.
- 16. ’üĮ Pyrolysis performed in the presence of Hydrogen. ’üĮ Application of suitable solvents and catalysts can speed up the reaction and increase the product yield. ’üĮ Very promising method for producing phenols from lignin. ŌŚ” Leads to higher net conversion, higher yields of monophenols, and less char formation. ’é¢ Yields can be increased even greater if pretreatment techniques such as microwave and/or ultrasound irradiation is performed before hydrogenolysis. ’üĮ Reaction temperature range is: 300-600 ┬░C. ’üĮ Performed by two different methods ŌŚ” Treating lignin to gaseous Hydrogen ŌŚ” Treating lignin to hydrogen donating solvent.
- 17. ’üĮ A method using gaseous hydrogen to depolymerize lignin is called Base Catalyzed Depolymerization (BCD). In general this method is used before hydrogenation to convert lignin to gasoline. ’üĮ Shabtai et al. proposed a multi step process for converting lignin into reformulated gasoline that includes BCD followed by hydrogenation and hydrocracking. ’üĮ The BCD reaction uses a catalyst-solvent system of an alkali hydroxide (KOH) and a supercritical alcohol. ŌŚ” Reaction Conditions: Temperatures around 270 ┬░C and pressure of 140 bar for 1-5 minutes ŌŚ” Reactions cause about a 50% reduction in Oxygen compared to native lignin.
- 18. ’üĮ After BCD the following stages occur to produce reformulated gasoline: ŌŚ” 2nd stage: Depolymerized lignin is subjected to hydrodeoxygenation (HDO) in the presence of catalyst. (ex. CoMo/Al2O3) ŌŚ” 3rd stage: Involves partial ring hydrogenation and mild hydrocracking in a hydrogen-rich catalytic environment. ŌŚ” Final product compares to reformulated gasoline. ŌŚ” If Chemicals are desired then the step can be skipped to produce phenolics.
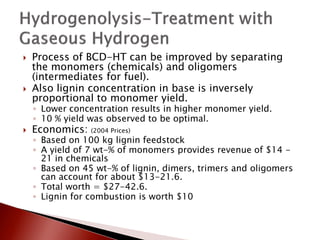
- 19. ’üĮ Process of BCD-HT can be improved by separating the monomers (chemicals) and oligomers (intermediates for fuel). ’üĮ Also lignin concentration in base is inversely proportional to monomer yield. ŌŚ” Lower concentration results in higher monomer yield. ŌŚ” 10 % yield was observed to be optimal. ’üĮ Economics: (2004 Prices) ŌŚ” Based on 100 kg lignin feedstock ŌŚ” A yield of 7 wt-% of monomers provides revenue of $14 - 21 in chemicals ŌŚ” Based on 45 wt-% of lignin, dimers, trimers and oligomers can account for about $13-21.6. ŌŚ” Total worth = $27-42.6. ŌŚ” Lignin for combustion is worth $10
- 20. ’üĮ Gasoline additives/blending agents can also be made in a similar fashion. ’üĮ Johnson et al. had a goal to convert lignin into a hydrocarbon product compatible with blending in gasoline that has an octane number greater than 110. ’üĮ BCD is first performed on lignin. Then it is isolated by acidification and then by solvent extraction (Diethyl Ether). Then catalytic hydroprocessing (HPR) is performed to convert the BCD lignin into aromatic hydrocarbons that can be blended with gasoline. ŌŚ” Production cost of BCD-HPR is $0.60-0.75/gallons ŌŚ” Value of lignin-derived gasoline blending component is $0.97-1.14/gallons
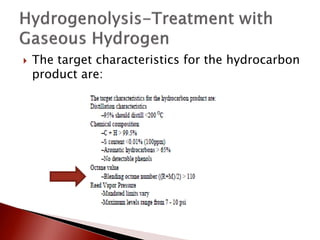
- 21. ’üĮ The target characteristics for the hydrocarbon product are:
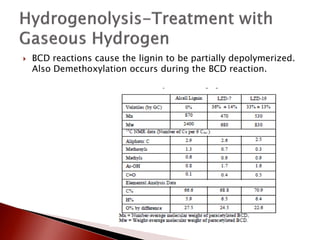
- 22. ’üĮ BCD reactions cause the lignin to be partially depolymerized. Also Demethoxylation occurs during the BCD reaction.
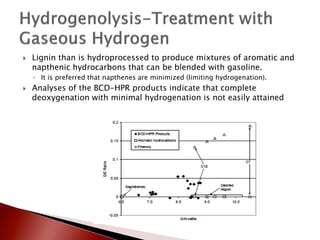
- 23. ’üĮ Lignin than is hydroprocessed to produce mixtures of aromatic and napthenic hydrocarbons that can be blended with gasoline. ŌŚ” It is preferred that napthenes are minimized (limiting hydrogenation). ’üĮ Analyses of the BCD-HPR products indicate that complete deoxygenation with minimal hydrogenation is not easily attained
- 24. ’üĮ Studies have also performed BCD-HT on different types of Isolated Lignin ŌŚ” Kraft Lignin, Organosolv Lignin, Ethanol Lignin ’üĮ Results show similar product spectrum and small differences in chemical reactivity ’üĮ Analysis of products from model compound reactions revealed that phenyl ether linkages were effectively broken while Carbon-Carbon linkages were less affected.
- 25. ’üĮ Using Tetralin as an Hydrogen Donating Solvent ŌŚ” Davoudxadeh et al. used tetralin with phenol for lignin hydrogenolysis to give greater liquid yields than neat pyrolysis. ’é¢ A yield of 20 wt-% was observed at 345 ┬░C ’üĮ Upon dehydrogenation, tetralin releases 4 Hydrogen atoms at hydrocracking severities and is converted to napthalene. ŌŚ” Therefore hydrocracking severities stops reaction. ŌŚ” Presence of tetralin for long residence times causes the reduction in the amount of guaiacol yield and increases the yield of phenol.
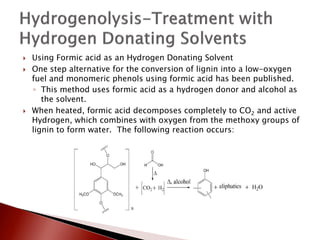
- 26. ’üĮ Using Formic acid as an Hydrogen Donating Solvent ’üĮ One step alternative for the conversion of lignin into a low-oxygen fuel and monomeric phenols using formic acid has been published. ŌŚ” This method uses formic acid as a hydrogen donor and alcohol as the solvent. ’üĮ When heated, formic acid decomposes completely to CO2 and active Hydrogen, which combines with oxygen from the methoxy groups of lignin to form water. The following reaction occurs:
- 27. ’üĮ Lignin is good for oxidation or oxidative cracking due to the presence of hydroxyl groups. ’üĮ The oxidative cracking involves the cleavage of the lignin rings, aryl ether bonds, or other linkages within the lignin. ’üĮ Products: Range from aromatic aldehydes to carboxylic acids based on the severity of the reaction conditions. ŌŚ” Softwood: vanillin (industrially produced), vanillic acid ŌŚ” Hardwood: syringaldehyde, syringic acid ’üĮ Nitrobenzene, metal oxides, and hydrogen peroxide (not good for aldehyde production) are the most popular oxidants for lignin, while catalytic oxidation with oxygen is also possible.
- 28. ’üĮ Solvents in supercritical conditions behave differently than in subcritical conditions. ŌŚ” Many advantages. Ex. Have ability to dissolve nonpolar organic molecules and inorganic solvents. ’üĮ Decomposition of Lignin in supercritical water occurs first by hydrolysis and then by dealkylation yielding low-molecular-weight fragments. ’üĮ Conversion of Lignin in Supercritical water has been studied ŌŚ” Conditions: T=374.15 ┬░C, P= 22.1 MPa ŌŚ” Monomeric yields have been low due to repolymerization into char ’é¢ Can increase yields by introducing phenols.
- 29. ’üĮ Catalysts used in lignin depolymerization should promote high conversion and suppress char formation and condensation. ŌŚ” Conditions are not severe ŌŚ” Also should assist in selective bond cleavage, leading to high selectivity values for particular compounds. ’üĮ Various catalysts are used for different processes and substrates. ŌŚ” Zeolites and amorphouse silica-alumina catalysts ’é¢ Disrupting the lignin polymer by cracking and upgrading pyrolysis oils. ’é¢ Zeolites produced more aromatic hydrocarbons ’é¢ Silica-alumina favors aliphatic hydrocarbons ŌŚ” Base catalysts (KOH and NaOH) ’é¢ effective in lignin hydrolysis (BCD) ŌŚ” Hydrogenation Catalysts (Co, W, Pd, Pt, Ni, RuŌĆ”) ’é¢ Increases the yield and promotes hydrodeoxygenation
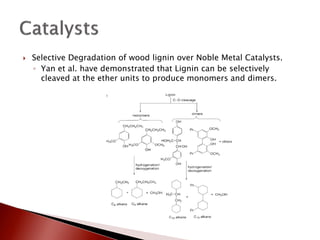
- 30. ’üĮ Selective Degradation of wood lignin over Noble Metal Catalysts. ŌŚ” Yan et al. have demonstrated that Lignin can be selectively cleaved at the ether units to produce monomers and dimers.
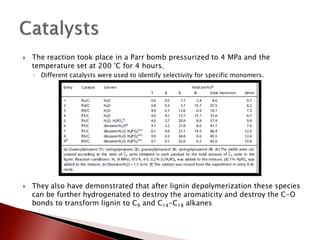
- 31. ’üĮ The reaction took place in a Parr bomb pressurized to 4 MPa and the temperature set at 200 ┬░C for 4 hours. ŌŚ” Different catalysts were used to identify selectivity for specific monomers. ’üĮ They also have demonstrated that after lignin depolymerization these species can be further hydrogenated to destroy the aromaticity and destroy the C-O bonds to transform lignin to C9 and C14-C18 alkanes
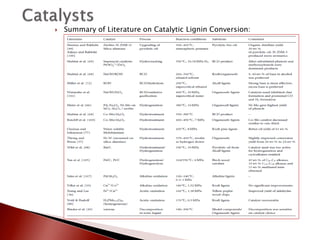
- 32. ’üĮ Summary of Literature on Catalytic Lignin Conversion: