04f Hydrogen Damage.ppt
- 1. uop Corrosion and Fouling Hydrogen Damage Jun 2002 1 Hydrogen Damage
- 2. uop Corrosion and Fouling Hydrogen Damage Jun 2002 2 Low Temp Hydrogen Damage • Several terms applied – Hydrogen blistering – Hydrogen induced cracking (HIC) – Stress oriented hydrogen induced cracking (SOHIC) – Sulfide stress cracking (SSC) or hydrogen stress cracking • All variations of same phenomenon
- 3. uop Corrosion and Fouling Hydrogen Damage Jun 2002 3 General Mechanism • Penetration of atomic hydrogen (Ho) into steel • At low temperatures (<400 F or 204C) molecular hydrogen does not react with steel and does not penetrate • Atomic hydrogen comes from any wet corrosion reactions which makes H2 – Often associated with H2S
- 4. uop Corrosion and Fouling Hydrogen Damage Jun 2002 4 General Mechanism Cont. • Atomic Hydrogen generated by corrosion normally combines to form molecular hydrogen and escapes into solution • Some doesn’t • Recombination poisons promote penetration – CN-, As, S2- – CN- also forms soluble complex with Fe
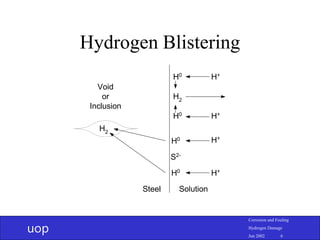
- 5. uop Corrosion and Fouling Hydrogen Damage Jun 2002 5 Hydrogen Blistering • Low tensile strength steel (high ductility) • Voids and inclusions – Particularly MnS stringers • Atomic hydrogen finds void and recombines to molecular hydrogen
- 6. uop Corrosion and Fouling Hydrogen Damage Jun 2002 6 Hydrogen Blistering S2- H+ H+ H+ H+ H0 H0 H0 H0 H2 H2 Void or Inclusion Steel Solution
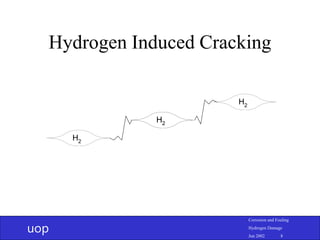
- 7. uop Corrosion and Fouling Hydrogen Damage Jun 2002 7 Hydrogen Induced Cracking • Starts at elongated MnS inclusions • Cracks start at tips of blisters and grow parallel to steel surface • Link by growing perpendicular • Want low Mn and S steel with shape controlled inclusions
- 8. uop Corrosion and Fouling Hydrogen Damage Jun 2002 8 Hydrogen Induced Cracking H2 H2 H2 H2
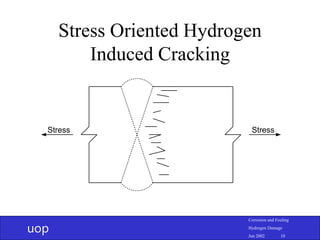
- 9. uop Corrosion and Fouling Hydrogen Damage Jun 2002 9 Stress Oriented Hydrogen Induced Cracking (SOHIC) • Starts from wet corrosion producing H0 which diffuses in • H0 collect at lattice defects but don’t necessarily form voids • Cracks form in plane of inclusions and link in plane perpendicular to stress • Often at hard spots near welds
- 10. uop Corrosion and Fouling Hydrogen Damage Jun 2002 10 Stress Oriented Hydrogen Induced Cracking Stress Stress
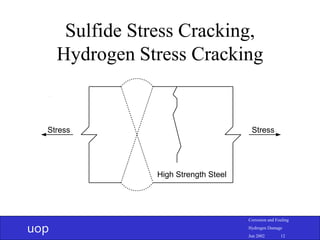
- 11. uop Corrosion and Fouling Hydrogen Damage Jun 2002 11 Sulfide Stress Cracking • High strength steel or hard welds • Stress or residual stress • Temperature and hydrogen flux affect cracking • Unlike other types of hydrogen damage significant wet corrosion is not required • Heat treat steel, welds < HRc 22 (HV 248)
- 12. uop Corrosion and Fouling Hydrogen Damage Jun 2002 12 Sulfide Stress Cracking, Hydrogen Stress Cracking Stress Stress High Strength Steel
- 13. uop Corrosion and Fouling Hydrogen Damage Jun 2002 13 Examples • HF units • Sour water strippers • FCC – HP and LP separators – Stripper tower overhead
- 14. uop Corrosion and Fouling Hydrogen Damage Jun 2002 14 What to do about it • Minimize wet corrosion (generates H0) • Minimize recombination poisons (H2S etc.) • Use low Mn, S steels with inclusion shape control • Avoid high strength steels • Heat treat welds to below HRc 22