1. Separation of Mixtures.pptx Grade 8 - Checkpoint
- 1. NAMING APPARATUS Learning objectives: ? To be able to able to name the commonly used apparatus in science
- 2. APPARATUS ? Use the worksheet on Lab equipment and skills (on Google Classroom) to complete the worksheet Apparatus in Science.
- 3. SEPARATION OF MIXTURES Learning objectives: ? To be able to apply different separation techniques to different examples of mixtures
- 4. STARTER ¡ In this jar is a mixture of iron filings, grains of sand and plastic beads. Suggest a way of isolating all three substances, so that they are on their own. Write this in your exercise book. You may have to use multiple steps. ANSWERS
- 5. Matter Pure Substance Impure substance (mixture) Element Compound Activity Draw the chart into your note book (we will define pure substances later on in the subject
- 6. WHAT IS A MIXTURE? ?A mixture is made when two or more substances are combined, but they are not chemically combined ?e.g. Air, salt and water, mixture of sand and iron ?The components of a mixture can be easily separated
- 7. WHY DOWE NEED TO SEPARATE MIXTURES? ? We separate mixtures so that substances can be purified ? Mixtures are usually easy to separate because the substances in the mixtures have different physical properties ? The key to separation is recognising the different properties of the substances that you are trying to separate
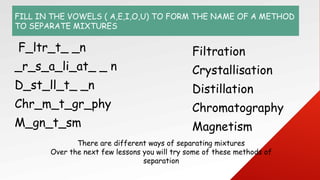
- 8. FILL IN THE VOWELS ( A,E,I,O,U) TO FORM THE NAME OF A METHOD TO SEPARATE MIXTURES F_ltr_t_ _n _r_s_a_li_at_ _ n D_st_ll_t_ _n Chr_m_t_gr_phy M_gn_t_sm Filtration Crystallisation Distillation Chromatography Magnetism There are different ways of separating mixtures Over the next few lessons you will try some of these methods of separation
- 9. WHAT DOYOU NOTICE WHEN? a) Sand is dissolved in water b) Salt is dissolved in water Insoluble Mixture Soluble Mixture
- 10. Filtration is used to separate an insoluble solid from a liquid for example, sand from water Residue ¨C is the solid component left in the filter paper Filtrate ¨C is the liquid component that passes through the filter paper FILTRATION
- 11. Crystallisation is used to separate a soluble solid from a liquid. For example, salt from water The liquid portion turns into a gas and evaporates off leaving the solid portion, residue, behind. CRYSTALLISATION
- 12. DEMO Video demonstrating simple distillation Distillation is a separating technique that separates liquids of different boiling points
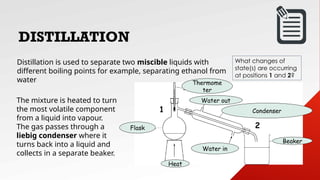
- 13. Distillation is used to separate two miscible liquids with different boiling points for example, separating ethanol from water The mixture is heated to turn the most volatile component from a liquid into vapour. The gas passes through a liebig condenser where it turns back into a liquid and collects in a separate beaker. Thermome ter Water out Water in Condenser Flask Heat Beaker 1 2 What changes of state(s) are occurring at positions 1 and 2? DISTILLATION
- 15. PRACTICAL: SEPARATING SALT AND SAND Apparatus Eye protection Beaker ¨C 250 cm3 Glass rod Filter funnel Filter paper Conical flask Evaporating basin Bunsen burner Heat resistant mat Tripod gauze Method 1. Pour the sand-salt mixture into the beaker so that it just covers the base 2. Add about 50 cm3 of water, or add water until the beakers is about 1/5th full 3. Stir the mixture gently for a few minutes 4. Filter the mixture into a conical flask 5. Pour the filtrate into an evaporating basin 6. Heat the salt solution gently until it starts to spit (decrepitate) 7. Turn off the Bunsen burner and let the damp salt dry in the dish Safety Wear eye protection throughout the experiment Chemicals 7g mixture of sand and salt
- 16. Copy out the questions and answer in your exercise book. 1. What substance was left behind in the filter paper? 2. What substance went through the filter paper? 3. Why can sand and salt be separated using this experiment? 4. Why is the salt, sand and water mixture stirred in step 3? 5. Why is the salt solution heated in step 6? 6. Give a reason why the sand you have obtained might still be contaminated with salt? PRACTICAL: QUESTIONS
- 17. 1. Sand 2. Salt water 3. Sand is insoluble and will be left in the filter paper (residue). Salt is soluble in water. 4. To dissolve the salt 5. So that the water will evaporate off leaving salt crystals behind 6. Some of the salt may not have dissolved whilst stirring PRACTICAL: ANSWERS
- 18. CHROMATOGRAPHY In this separation technique we can determine what chemicals are in a solution. This is used in forensic science to determine who committed crimes (you¡¯ll see how in the video). The different-size particles will travel through the chromatography paper at different speeds and so they separate out at different distances
- 19. CHROMATOGRAPHY ?Chromatography can be used to separate mixtures of different colours ?Mixtures that are suitable for separation by chromatography include inks, dyes and colouring agents in foods.
- 20. CHROMATOGRAPHY If the conditions for developing a chromatogram are the same, then the distance a particular substance moves should not change. This distance is measured by the retention factor formula or Rf Task: Calculate the Rf value for the lilac component
- 21. HOW TO DO CHROMATOGRAPHY
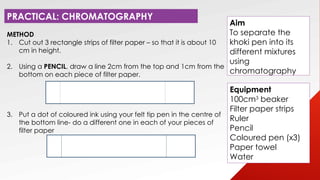
- 22. METHOD 1. Cut out 3 rectangle strips of filter paper ¨C so that it is about 10 cm in height. 2. Using a PENCIL, draw a line 2cm from the top and 1cm from the bottom on each piece of filter paper. 3. Put a dot of coloured ink using your felt tip pen in the centre of the bottom line- do a different one in each of your pieces of filter paper Equipment 100cm3 beaker Filter paper strips Ruler Pencil Coloured pen (x3) Paper towel Water PRACTICAL: CHROMATOGRAPHY Aim To separate the khoki pen into its different mixtures using chromatography
- 23. Filter paper chromatogram. Beaker Splint/Pencil Fill with water so that it just touches the dot. 4. Set up your chromatogram (the filter paper strip) in the beaker as in the diagram below: 5. Wait for the water to reach the top line and then remove the filter paper from the splint and lay it on some paper towel to dry. PRACTICAL: CHROMATOGRAPHY
- 24. Copy out and answer the following questions: 1) Which ink contains the most different colours? 2) How do you know this? 3) For one of the inks on your chromatogram calculate the Rf values for each colour PRACTICAL: CHROMATOGRAPHY RESULTS ¡ñ Stick the three chromatograms in your book ¡ñ State the two (or three) colours that you used for chromatography
- 25. Ink from crime scene Ink A Ink B Ink C Ink D A crime has been committed- another teacher has stolen Ms Smith¡¯s lunch. Luckily some ink was left behind, so can you work out who stole my lunch? Ink A= Mr Luthi Ink B= Mrs Rue Ink C= Mr Malinga Ink D= Ms Ndlovu WHO STOLE LUNCH?
- 26. Ink from crime scene Ink A Ink B Ink C Ink D A crime has been committed- another teacher has stolen Ms Smith¡¯s lunch. Luckily some ink was left behind, so can you work out who stole my lunch? WHO STOLE LUNCH? Mr Malinga Ink A= Mr Luthi Ink B= Mrs Rue Ink C= Mr Malinga Ink D= Ms Ndlovu
Editor's Notes
- #4: Answers: Magnet ¨C iron filings will attach to the magnet Sieve ¨C choosing a sieve where the plastic beads are too big to pass through Magnet ¨C iron filings will attach to the magnet Sieve ¨C choosing a sieve where the plastic beads are too big to pass through
- #5: Teacher?s notes: Ask students to define what matter is
- #10: Teacher¡¯s notes Students are to draw filtration using scientific drawing rules
- #11: Teacher¡¯s notes Students are to draw filtration using scientific drawing rules
- #12: Teacher¡¯s notes Probably still won¡¯t have distillation kit and so show them this technique and talk them through it
- #13: Teacher¡¯s notes Only copy out the notes, students will get diagram on the worksheet on the following slide
- #15: DEMONSTRATE how to fold the filter paper Print the practical for yourself - RSC
- #18: Teacher¡¯s notes Cool video of a CSI clip where they can see that there were two different inks that were used in the sample
- #21: Teacher¡¯s notes: 3 minute video on how to do chromatography
- #22: Teacher¡¯s notes: