10/12 Review: What is atomic radius?
0 likes1,825 views
The document contains data from multiple quizzes and exams, showing the class's goal score of 80% and their actual scores just below the goal. It also contains information about an upcoming mid-term exam covering three units of material and notes the need to ask questions if anything is not understood to improve scores. The document discusses trends in atomic radius, explaining that radius decreases left to right across periods and increases top to bottom in groups on the periodic table. It includes examples comparing elements to determine which has a smaller or larger atomic radius.
1 of 18
Downloaded 26 times















Recommended
Atomic radius ppt for chem



Atomic radius ppt for chemroblmcca13
╠²
The document discusses several atomic properties including atomic radius, ionic radius, ionization energy, electronegativity, nuclear charge, and shielding effect. It provides trends within the periodic table, noting that atomic radius increases down a group and decreases left to right across a period. Ionic radius also decreases left to right within a period and increases down a group. Ionization energy increases left to right within a period and decreases down a group. Electronegativity increases left to right within a period and decreases down a group. The document also examines nuclear charge and shielding effect.3 2 What Is Heat



3 2 What Is Heatmrheffner
╠²
The document outlines a science lesson on heat and phase changes. It provides objectives for defining heat and classifying phase changes as endothermic or exothermic. Examples of endothermic and exothermic processes like melting, evaporation, condensation and freezing are discussed. Students complete exit slips to assess their understanding.2 24 Unit #7 Jeopardy



2 24 Unit #7 Jeopardymrheffner
╠²
The document provides an agenda and materials for a chemistry class review session in preparation for an exam on Unit 7. The session will include reviewing practice questions from the previous day, going over the objectives for the day which is a review, and participating in a jeopardy game to further review the material in a fun way. The calendar shows that today is the review session and tomorrow will be the exam.2 23 Gases Review



2 23 Gases Reviewmrheffner
╠²
This document provides an agenda and review for an exam on gases. It includes directions for students, data from an exit slip, learning objectives, example problems on gas laws, and an exit slip for students. The objective is to review concepts related to gases, kinetic molecular theory, temperature, pressure, volume, and the combined gas law in preparation for an upcoming exam.2 22 What Is The Combined Gas Law



2 22 What Is The Combined Gas Lawmrheffner
╠²
The document contains instructions and objectives for a chemistry class. It provides the date of February 22nd and instructs students to take their seats, get out their binders and yesterday's practice questions. It then shares the results from an exit slip on Friday, ranging from 88% to 97%. The objective for the day is to apply the combined gas law to relationships between temperature, pressure and volume.2 10 How Are P And T Related



2 10 How Are P And T Relatedmrheffner
╠²
The document provides information about a chemistry lesson on gas laws and the relationship between temperature and pressure. It includes objectives, examples, and questions for students. The lesson explains that temperature and pressure are directly related, as temperature increases so does molecular motion and collisions, thus increasing pressure. Examples show how to use the direct relationship equation to calculate new pressure or temperature given one variable.2 9 Chemical Bonding Benchmark Review Part Ii



2 9 Chemical Bonding Benchmark Review Part Iimrheffner
╠²
The document provides an agenda and objectives for a chemistry lesson on chemical bonding. It includes steps to draw Lewis structures for different molecules like carbon, chlorine, ammonia, and methane. It reviews ionic and covalent bonding between elements. Students complete an exit slip with multiple choice questions and have a review worksheet for homework to study for an upcoming exam on chemical bonding.2 4 What Is Pressure



2 4 What Is Pressuremrheffner
╠²
The document instructs students to get their binders and take a seat. It reports that 96% of students answered yesterday's practice questions correctly. The objectives for the lesson are to describe how temperature relates to molecular motion and to convert between the Kelvin and Celsius temperature scales.2 3 What Is Temperature



2 3 What Is Temperaturemrheffner
╠²
The document provides instructions for students on starting their science lesson on molecular motion and temperature. It tells students to take their seats, get out their binders and yesterday's work, and notes that 96% of students understood yesterday's lesson based on exit slip data. The objectives for today's lesson are described as learning how temperature relates to molecular motion and how to convert between the Kelvin and Celsius temperature scales.1 27 Unit #6 Jeopardy



1 27 Unit #6 Jeopardymrheffner
╠²
The document provides a lesson plan and materials for a chemistry class reviewing solutions, acids, and bases. It includes:
1) A jeopardy game for students to review concepts with questions about solutions, acids, bases, and dissolving processes.
2) Announcements that the unit 6 exam will be the next day without notes and that the goal is to review.
3) Sample jeopardy questions about solutions, concentrations, acids, bases, and chemical reactions.
4) Properties of acids and the definition of a Bronsted-Lowry base.1 27 Unit #6 Jeopardy



1 27 Unit #6 Jeopardymrheffner
╠²
The document provides a lesson plan and materials for a chemistry class reviewing solutions, acids, and bases. It includes:
1) A jeopardy game for students to review concepts with questions about solutions, acids, bases, and dissolving processes.
2) Announcements that the unit 6 exam will be the next day without notes and that the goal is to review.
3) Sample jeopardy questions about solutions, concentrations, acids, bases, and chemical reactions.
4) Properties of acids and the definition of a Bronsted-Lowry base.1 26 Unit #6 Exam Review



1 26 Unit #6 Exam Reviewmrheffner
╠²
The document provides information and instructions for a class. It includes:
- Yesterday's exit slip data showed 95-99% of students understanding a topic.
- Today's objective is to review for an upcoming exam on Thursday through working on a study guide and jeopardy game.
- The teacher emphasizes studying purposefully and asks students to focus on following class rules around interfering with learning and leaving seats without permission.1 22 What Are Strong Acids And Bases



1 22 What Are Strong Acids And Basesmrheffner
╠²
The document provides information about a chemistry lesson on strong and weak acids and bases according to the Br├Ėnsted-Lowry definition. It begins with an agenda and objectives, followed by practice questions on acids and bases. Definitions are then given for strong acids/bases, which fully dissociate, and weak acids/bases, which partially dissociate. An example reaction of a strong acid and base neutralizing to form water and salt is also given. The document concludes with an exit slip and homework assignment.1 25 What Is P H



1 25 What Is P Hmrheffner
╠²
This document provides instructions and information for a chemistry class. It notes that the class scored 94% on a recent quiz and that today's objective is to learn about using the pH scale to characterize acids and bases. It includes sample practice questions, instructions to organize binders, and an exit slip quiz on pH concepts.1 14 What Is Concentration Part Iii



1 14 What Is Concentration Part Iiimrheffner
╠²
This document provides instructions and information for a chemistry class. Students are told to take their seats and get out their homework and launch paper. Yesterday's exit slip data is provided, showing a 90% success rate. Today's objective is to learn how to calculate the concentration of a solution in parts per million (ppm). Examples are then provided for calculating ppm concentrations in solutions containing various amounts of solute and solvent. Key concepts around solutes, solvents, and concentration calculations are reviewed. Students are then instructed to work on practice concentration calculation problems by picking a question card and solving it.1 12 What Is Concentration Part Ii



1 12 What Is Concentration Part Iimrheffner
╠²
The document outlines the agenda for a class on calculating molarity, including having students take their seats, get out homework from the previous day, and states the learning objective is to calculate the concentration of a solution in terms of molarity. It provides no data from the previous day's exit slips. Students are instructed to calculate the concentration of solutions in terms of molarity.1 11 What Is Concentration



1 11 What Is Concentrationmrheffner
╠²
This document contains the agenda and materials for a science class lesson on concentration. The teacher provides instructions for students to take their seats and get out their homework. Exit slip data from the previous day is reported, ranging from 77-93%. The learning objective for the day is stated as being able to describe the factors that affect the dissolving process. The document then includes sample problems, examples, practice questions, and an exit slip for students to complete to assess their understanding of concentration.1 7 What Is Solubility



1 7 What Is Solubilitymrheffner
╠²
This document provides information about a science lesson on solubility. It discusses how solubility is defined as how well a solute dissolves in a given amount of solvent. It also describes two key factors that affect solubility - temperature and surface area for solids, and temperature and pressure for gases. Students are provided practice questions to work through, and an exit slip assessment to complete.1 6 How Are Solutes Dissolved



1 6 How Are Solutes Dissolvedmrheffner
╠²
This document contains the notes from a chemistry class lesson on solutions and the dissolving process. It defines key terms like solute and solvent. It then describes how solvent molecules randomly collide with and overcome the attractive forces between particles of the solute, allowing it to dissolve. Examples used sodium chloride dissolving in water. Students had an activity acting this out and completed practice questions and an exit slip assessment. The objective was for students to be able to describe the dissolving process at a molecular level.1 5 What Are Solutions



1 5 What Are Solutionsmrheffner
╠²
The document provides instructions for students on the first day of class. It instructs students to take a seat, write their name and block number on a folder, and organize any contents from their binder into the folder. It then states the objective of describing the three states of matter. The rest of the document outlines new policies for late homework and class consequences, and reminds students that the seven rules remain the same.1 5 What Are Solutions



1 5 What Are Solutionsmrheffner
╠²
The document provides instructions for students on the first day of class. It instructs students to take a seat, write their name and block number on a folder, and organize any contents from their binder into the folder. It then lists the objective for the class - to describe the three states of matter. It provides some new information about late homework policies and class consequences. 1 4 What Are The 3 States Of Matter



1 4 What Are The 3 States Of Mattermrheffner
╠²
The document provides information about the first day of chemistry class. It introduces the objectives to describe the three states of matter and their properties. It outlines the class rules, late homework policy, and where to find course materials online or in the classroom. It then describes the key properties of gases, liquids, and solids in relation to molecular movement and intermolecular attractions. Examples are given for each state. Students do an activity with Oobleck to determine if it is a solid, liquid, or gas, and take an exit slip quiz about the three states of matter.12 16 Mole Review Part Ii



12 16 Mole Review Part Iimrheffner
╠²
I apologize, upon reviewing the document again I do not see enough context to summarize it in 3 sentences or less. The document provides some details about an upcoming exam and homework assignments but does not have an overall topic or theme that can be succinctly summarized. Please provide more context if you would like me to attempt a summary.12 15 Mole Review



12 15 Mole Reviewmrheffner
╠²
Here are the steps to solve this problem:
1) Given: 12.04 X 1023 molecules of H2O
2) Conversion factors:
1 mol H2O / 6.02 X 1023 molecules H2O
18.02 g H2O / 1 mol H2O
3) Calculations:
12.04 X 1023 molecules H2O x (1 mol H2O / 6.02 X 1023 molecules H2O) x (18.02 g H2O / 1 mol H2O) = 36.04 g
The answer is d.12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains notes from a chemistry class that include:
- Reviewing how to balance chemical equations by counting atoms on each side, adjusting coefficients, and checking the balance
- Examples of balancing equations step-by-step
- Announcements about an upcoming exam on balancing equations and conversions between moles, grams, and atoms
- A homework assignment to finish a practice questions worksheet on balancing equations12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains information from a chemistry class, including:
- A practice exam average of 87% was achieved, with questions 5, 6, 7, 9, and 14 dropped.
- An upcoming unit exam on Thursday will cover balancing equations, the mole concept, and conversions between grams, moles, atoms, and grams.
- A snowman challenge activity had students work in groups to balance sample chemical equations.
- An exit slip posed sample chemistry problems related to balancing equations and stoichiometry.12 14 Balancing Review1



12 14 Balancing Review1mrheffner
╠²
The document contains information from a chemistry class, including:
- A practice exam average of 87% was achieved, with questions 5, 6, 7, 9, and 14 dropped.
- An upcoming unit exam on Thursday will cover balancing equations, the mole concept, and conversions between grams, moles, atoms, and grams.
- A snowman challenge activity had students work in groups to balance sample chemical equations.
- An exit slip posed sample questions testing the concepts of identifying reactants, calculating atoms in a formula, balancing equations, and determining missing coefficients.12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains information from a chemistry class, including:
- Instructions to get materials out for the lesson on balancing chemical equations.
- The objective is to learn how to write balanced chemical equations.
- Homework assigned is to finish a review worksheet on balancing equations.
- Sample equations are provided to demonstrate the three-step process to balance equations: counting atoms on each side, adjusting coefficients, and verifying the balanced tally.Supercharge Your Career with UiPath Certifications



Supercharge Your Career with UiPath CertificationsDianaGray10
╠²
Join us on February 25th as we discuss how you can supercharge your career with the updated 2025 UiPath Certifications.
Diana Gray, UiPath Senior Community Marketing Manager, Americas, will walk us through:
-- Workforce Trends
-- Value of UiPath Certifications
-- Certification Program
-- Steps to Earning a CertificateMore Related Content
More from mrheffner (20)
2 3 What Is Temperature



2 3 What Is Temperaturemrheffner
╠²
The document provides instructions for students on starting their science lesson on molecular motion and temperature. It tells students to take their seats, get out their binders and yesterday's work, and notes that 96% of students understood yesterday's lesson based on exit slip data. The objectives for today's lesson are described as learning how temperature relates to molecular motion and how to convert between the Kelvin and Celsius temperature scales.1 27 Unit #6 Jeopardy



1 27 Unit #6 Jeopardymrheffner
╠²
The document provides a lesson plan and materials for a chemistry class reviewing solutions, acids, and bases. It includes:
1) A jeopardy game for students to review concepts with questions about solutions, acids, bases, and dissolving processes.
2) Announcements that the unit 6 exam will be the next day without notes and that the goal is to review.
3) Sample jeopardy questions about solutions, concentrations, acids, bases, and chemical reactions.
4) Properties of acids and the definition of a Bronsted-Lowry base.1 27 Unit #6 Jeopardy



1 27 Unit #6 Jeopardymrheffner
╠²
The document provides a lesson plan and materials for a chemistry class reviewing solutions, acids, and bases. It includes:
1) A jeopardy game for students to review concepts with questions about solutions, acids, bases, and dissolving processes.
2) Announcements that the unit 6 exam will be the next day without notes and that the goal is to review.
3) Sample jeopardy questions about solutions, concentrations, acids, bases, and chemical reactions.
4) Properties of acids and the definition of a Bronsted-Lowry base.1 26 Unit #6 Exam Review



1 26 Unit #6 Exam Reviewmrheffner
╠²
The document provides information and instructions for a class. It includes:
- Yesterday's exit slip data showed 95-99% of students understanding a topic.
- Today's objective is to review for an upcoming exam on Thursday through working on a study guide and jeopardy game.
- The teacher emphasizes studying purposefully and asks students to focus on following class rules around interfering with learning and leaving seats without permission.1 22 What Are Strong Acids And Bases



1 22 What Are Strong Acids And Basesmrheffner
╠²
The document provides information about a chemistry lesson on strong and weak acids and bases according to the Br├Ėnsted-Lowry definition. It begins with an agenda and objectives, followed by practice questions on acids and bases. Definitions are then given for strong acids/bases, which fully dissociate, and weak acids/bases, which partially dissociate. An example reaction of a strong acid and base neutralizing to form water and salt is also given. The document concludes with an exit slip and homework assignment.1 25 What Is P H



1 25 What Is P Hmrheffner
╠²
This document provides instructions and information for a chemistry class. It notes that the class scored 94% on a recent quiz and that today's objective is to learn about using the pH scale to characterize acids and bases. It includes sample practice questions, instructions to organize binders, and an exit slip quiz on pH concepts.1 14 What Is Concentration Part Iii



1 14 What Is Concentration Part Iiimrheffner
╠²
This document provides instructions and information for a chemistry class. Students are told to take their seats and get out their homework and launch paper. Yesterday's exit slip data is provided, showing a 90% success rate. Today's objective is to learn how to calculate the concentration of a solution in parts per million (ppm). Examples are then provided for calculating ppm concentrations in solutions containing various amounts of solute and solvent. Key concepts around solutes, solvents, and concentration calculations are reviewed. Students are then instructed to work on practice concentration calculation problems by picking a question card and solving it.1 12 What Is Concentration Part Ii



1 12 What Is Concentration Part Iimrheffner
╠²
The document outlines the agenda for a class on calculating molarity, including having students take their seats, get out homework from the previous day, and states the learning objective is to calculate the concentration of a solution in terms of molarity. It provides no data from the previous day's exit slips. Students are instructed to calculate the concentration of solutions in terms of molarity.1 11 What Is Concentration



1 11 What Is Concentrationmrheffner
╠²
This document contains the agenda and materials for a science class lesson on concentration. The teacher provides instructions for students to take their seats and get out their homework. Exit slip data from the previous day is reported, ranging from 77-93%. The learning objective for the day is stated as being able to describe the factors that affect the dissolving process. The document then includes sample problems, examples, practice questions, and an exit slip for students to complete to assess their understanding of concentration.1 7 What Is Solubility



1 7 What Is Solubilitymrheffner
╠²
This document provides information about a science lesson on solubility. It discusses how solubility is defined as how well a solute dissolves in a given amount of solvent. It also describes two key factors that affect solubility - temperature and surface area for solids, and temperature and pressure for gases. Students are provided practice questions to work through, and an exit slip assessment to complete.1 6 How Are Solutes Dissolved



1 6 How Are Solutes Dissolvedmrheffner
╠²
This document contains the notes from a chemistry class lesson on solutions and the dissolving process. It defines key terms like solute and solvent. It then describes how solvent molecules randomly collide with and overcome the attractive forces between particles of the solute, allowing it to dissolve. Examples used sodium chloride dissolving in water. Students had an activity acting this out and completed practice questions and an exit slip assessment. The objective was for students to be able to describe the dissolving process at a molecular level.1 5 What Are Solutions



1 5 What Are Solutionsmrheffner
╠²
The document provides instructions for students on the first day of class. It instructs students to take a seat, write their name and block number on a folder, and organize any contents from their binder into the folder. It then states the objective of describing the three states of matter. The rest of the document outlines new policies for late homework and class consequences, and reminds students that the seven rules remain the same.1 5 What Are Solutions



1 5 What Are Solutionsmrheffner
╠²
The document provides instructions for students on the first day of class. It instructs students to take a seat, write their name and block number on a folder, and organize any contents from their binder into the folder. It then lists the objective for the class - to describe the three states of matter. It provides some new information about late homework policies and class consequences. 1 4 What Are The 3 States Of Matter



1 4 What Are The 3 States Of Mattermrheffner
╠²
The document provides information about the first day of chemistry class. It introduces the objectives to describe the three states of matter and their properties. It outlines the class rules, late homework policy, and where to find course materials online or in the classroom. It then describes the key properties of gases, liquids, and solids in relation to molecular movement and intermolecular attractions. Examples are given for each state. Students do an activity with Oobleck to determine if it is a solid, liquid, or gas, and take an exit slip quiz about the three states of matter.12 16 Mole Review Part Ii



12 16 Mole Review Part Iimrheffner
╠²
I apologize, upon reviewing the document again I do not see enough context to summarize it in 3 sentences or less. The document provides some details about an upcoming exam and homework assignments but does not have an overall topic or theme that can be succinctly summarized. Please provide more context if you would like me to attempt a summary.12 15 Mole Review



12 15 Mole Reviewmrheffner
╠²
Here are the steps to solve this problem:
1) Given: 12.04 X 1023 molecules of H2O
2) Conversion factors:
1 mol H2O / 6.02 X 1023 molecules H2O
18.02 g H2O / 1 mol H2O
3) Calculations:
12.04 X 1023 molecules H2O x (1 mol H2O / 6.02 X 1023 molecules H2O) x (18.02 g H2O / 1 mol H2O) = 36.04 g
The answer is d.12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains notes from a chemistry class that include:
- Reviewing how to balance chemical equations by counting atoms on each side, adjusting coefficients, and checking the balance
- Examples of balancing equations step-by-step
- Announcements about an upcoming exam on balancing equations and conversions between moles, grams, and atoms
- A homework assignment to finish a practice questions worksheet on balancing equations12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains information from a chemistry class, including:
- A practice exam average of 87% was achieved, with questions 5, 6, 7, 9, and 14 dropped.
- An upcoming unit exam on Thursday will cover balancing equations, the mole concept, and conversions between grams, moles, atoms, and grams.
- A snowman challenge activity had students work in groups to balance sample chemical equations.
- An exit slip posed sample chemistry problems related to balancing equations and stoichiometry.12 14 Balancing Review1



12 14 Balancing Review1mrheffner
╠²
The document contains information from a chemistry class, including:
- A practice exam average of 87% was achieved, with questions 5, 6, 7, 9, and 14 dropped.
- An upcoming unit exam on Thursday will cover balancing equations, the mole concept, and conversions between grams, moles, atoms, and grams.
- A snowman challenge activity had students work in groups to balance sample chemical equations.
- An exit slip posed sample questions testing the concepts of identifying reactants, calculating atoms in a formula, balancing equations, and determining missing coefficients.12 14 Balancing Review



12 14 Balancing Reviewmrheffner
╠²
The document contains information from a chemistry class, including:
- Instructions to get materials out for the lesson on balancing chemical equations.
- The objective is to learn how to write balanced chemical equations.
- Homework assigned is to finish a review worksheet on balancing equations.
- Sample equations are provided to demonstrate the three-step process to balance equations: counting atoms on each side, adjusting coefficients, and verifying the balanced tally.Recently uploaded (20)
Supercharge Your Career with UiPath Certifications



Supercharge Your Career with UiPath CertificationsDianaGray10
╠²
Join us on February 25th as we discuss how you can supercharge your career with the updated 2025 UiPath Certifications.
Diana Gray, UiPath Senior Community Marketing Manager, Americas, will walk us through:
-- Workforce Trends
-- Value of UiPath Certifications
-- Certification Program
-- Steps to Earning a CertificateMastering ChatGPT & LLMs for Practical Applications: Tips, Tricks, and Use Cases



Mastering ChatGPT & LLMs for Practical Applications: Tips, Tricks, and Use CasesSanjay Willie
╠²
Our latest session with Astiostech covered how to unlock the full potential of ChatGPT and LLMs for real-world use!
Ō£ģ Key Takeaways:
¤ö╣ Effective Prompting: Crafting context-specific, high-quality prompts for optimal AI responses.
¤ö╣ Advanced ChatGPT Features: Managing system prompts, conversation memory, and file uploads.
¤ö╣ Optimizing AI Outputs: Refining responses, handling large texts, and knowing when fine-tuning is needed.
¤ö╣ Competitive Insights: Exploring how ChatGPT compares with other AI tools.
¤ö╣ Business & Content Use Cases: From summarization to SEO, sales, and audience targeting.
¤ÆĪ The session provided hands-on strategies to make AI a powerful tool for content creation, decision-making, and business growth.
¤ÜĆ Are you using AI effectively in your workflow? LetŌĆÖs discuss how it can improve efficiency and creativity!
#AI #ChatGPT #PromptEngineering #ArtificialIntelligence #LLM #Productivity #AstiostechData-Driven Public Safety: Reliable Data When Every Second Counts



Data-Driven Public Safety: Reliable Data When Every Second CountsSafe Software
╠²
When every second counts, you need access to data you can trust. In this webinar, weŌĆÖll explore how FME empowers public safety services to streamline their operations and safeguard communities. This session will showcase workflow examples that public safety teams leverage every day.
WeŌĆÖll cover real-world use cases and demo workflows, including:
Automating Police Traffic Stop Compliance: Learn how the City of Fremont meets traffic stop data standards by automating QA/QC processes, generating error reports ŌĆō saving over 2,800 hours annually on manual tasks.
Anonymizing Crime Data: Discover how cities protect citizen privacy while enabling transparent and trustworthy open data sharing.
Next Gen 9-1-1 Integration: Explore how Santa Clara County supports the transition to digital emergency response systems for faster, more accurate dispatching, including automated schema mapping for address standardization.
Extreme Heat Alerts: See how FME supports disaster risk management by automating the delivery of extreme heat alerts for proactive emergency response.
Our goal is to provide practical workflows and actionable steps you can implement right away. Plus, weŌĆÖll provide quick steps to find more information about our public safety subscription for Police, Fire Departments, EMS, HAZMAT teams, and more.
Whether youŌĆÖre in a call center, on the ground, or managing operations, this webinar is crafted to help you leverage data to make informed, timely decisions that matter most.Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...



Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...Earley Information Science
╠²
Revolutionizing Field Service with LLM-Powered Knowledge Management
Field service technicians need instant access to accurate repair information, but outdated knowledge systems often create frustrating delays. Large Language Models (LLMs) are changing the gameŌĆöenhancing knowledge retrieval, streamlining troubleshooting, and reducing technician dependency on senior staff.
In this webinar, Seth Earley and industry experts Sanjay Mehta, and Heather Eisenbraun explore how LLMs and Retrieval-Augmented Generation (RAG) are transforming field service operations. Discover how AI-powered knowledge management is improving efficiency, reducing downtime, and elevating service quality.
LLMs for Instant Knowledge Retrieval ŌĆō How AI-driven search dramatically cuts troubleshooting time.
Structured Data & AI ŌĆō Why high-quality, organized knowledge is essential for LLM success.
Real-World Implementation ŌĆō Lessons from deploying LLM-powered knowledge tools in field service.
Business Impact ŌĆō How AI reduces service delays, optimizes workflows, and enhances technician productivity.
Empower your field service teams with AI-driven knowledge access. Watch the webinar to see how LLMs are revolutionizing service efficiency.Leadership u automatizaciji: RPA pri─Źe iz prakse!



Leadership u automatizaciji: RPA pri─Źe iz prakse!UiPathCommunity
╠²
Dobrodo┼Īli na "AI Powered Automation Leadership Talks", online doga─æaj koji okuplja senior lidere i menad┼Šere iz razli─Źitih industrija kako bi podelili svoja iskustva, izazove i strategije u oblasti RPA (Robotic Process Automation). Ovaj doga─æaj pru┼Ša priliku da zavirite u na─Źin razmi┼Īljanja ljudi koji donose klju─Źne odluke u automatizaciji i liderstvu.
¤ōĢ Kroz panel diskusiju sa tri izuzetna stru─Źnjaka, istra┼Ši─ćemo:
Kako uspe┼Īno zapo─Źeti i skalirati RPA projekte u organizacijama.
Koji su najve─ći izazovi u implementaciji RPA-a i kako ih prevazi─ći.
Na koje na─Źine automatizacija menja radne procese i poma┼Še timovima da ostvare vi┼Īe.
Bez obzira na va┼Īe iskustvo sa UiPath-om ili RPA uop┼Īte, ovaj doga─æaj je osmi┼Īljen kako bi bio koristan svima ŌĆō od menad┼Šera do tehni─Źkih lidera, i svima koji ┼Šele da unaprede svoje razumevanje automatizacije.
Pridru┼Šite nam se i iskoristite ovu jedinstvenu priliku da nau─Źite od onih koji vode automatizaciju u svojim organizacijama. Pripremite svoja pitanja i inspiraciju za slede─će korake u va┼Īoj RPA strategiji!Understanding & Utilizing SharePoint Advanced Management



Understanding & Utilizing SharePoint Advanced ManagementDrew Madelung
╠²
Drew Madelung is a Cloud Solutions Architect and a Microsoft MVP for Office Apps and Services. He helps organizations realize what is possible with Microsoft 365 & Azure, onboard them in a secure and compliant way, and drive sustained adoption for those solutions. He is experienced in a range of technologies but specializes in the collaboration and teamwork workspaces such as Microsoft Teams, SharePoint, and OneDrive. He has helped deploy Microsoft 365 to multiple global companies while rolling out modern information protection and information governance technologies. He has been doing Microsoft consulting for 10+ years with a strength in security & compliance solutions.Build with AI on Google Cloud Session #3



Build with AI on Google Cloud Session #3Margaret Maynard-Reid
╠²
This is session #3 of the 5-session online study series with Google Cloud, where we take you onto the journey learning generative AI. YouŌĆÖll explore the dynamic landscape of Generative AI, gaining both theoretical insights and practical know-how of Google Cloud GenAI tools such as Gemini, Vertex AI, AI agents and Imagen 3. Webinar: LF Energy GEISA: Addressing edge interoperability at the meter



Webinar: LF Energy GEISA: Addressing edge interoperability at the meterDanBrown980551
╠²
This webinar will introduce the Grid Edge Security and Interoperability Alliance, or GEISA, an effort within LF Energy to address application interoperability at the very edge of the utility network: meters and other distribution automation devices. Over the last decade platform manufacturers have introduced the ability to run applications on electricity meters and other edge devices. Unfortunately, while many of these efforts have been built on Linux, they havenŌĆÖt been interoperable. APIs and execution environment have varied from one manufacturer to the next making it impossible for utilities to obtain applications that they can run across a fleet of different devices. For utilities that want to minimize their supply chain risk by obtaining equipment from multiple suppliers, they are forced to run and maintain multiple separate management systems. Applications available for one device may need to be ported to run on another, or they may not be available at all.
GEISA addresses this by creating a vendor neutral specification for utility edge computing environments. This webinar will discuss why GEISA is important to utilities, the specific issues GEISA will solve and the new opportunities it creates for utilities, platform vendors, and application vendors. Not a Kubernetes fan? The state of PaaS in 2025



Not a Kubernetes fan? The state of PaaS in 2025Anthony Dahanne
╠²
Kubernetes won the containers orchestration war. But has it made deploying your apps easier?
Let's explore some of Kubernetes extensive app developer tooling, but mainly what the PaaS space looks like in 2025; 18 years after Heroku made it popular.
Is Heroku still around? What about Cloud Foundry?
And what are those new comers (fly.io, railway, porter.sh, etc.) worth?
Did the Cloud giants replace them all?5 Must-Use AI Tools to Supercharge Your Productivity



5 Must-Use AI Tools to Supercharge Your Productivitycryptouniversityoffi
╠²
5 Must-Use AI Tools to Supercharge Your Productivity!
AI is changing the game! ¤ÜĆ From research to creativity and coding, here are 5 powerful AI tools you should try.
NotebookLM
¤ōÜ NotebookLM ŌĆō Your AI Research Assistant
Ō£ģ Organizes & summarizes notes
Ō£ģ Generates insights from multiple sources
Ō£ģ Ideal for students, researchers & writers
¤ōØ Boost your productivity with smarter note-taking!
Napkin.ai
¤Ä© Napkin.ai ŌĆō The Creativity Booster
Ō£ģ Connects and organizes ideas
Ō£ģ Perfect for writers, designers & entrepreneurs
Ō£ģ Acts as your AI-powered brainstorming partner
¤ÆĪ Unleash your creativity effortlessly!
DeepSeek
¤öŹ DeepSeek ŌĆō Smarter AI Search
Ō£ģ Delivers deeper & more precise search results
Ō£ģ Analyzes large datasets for better insights
Ō£ģ Ideal for professionals & researchers
¤öÄ Find what you needŌĆöfaster & smarter!
ChatGPT
¤Æ¼ ChatGPT ŌĆō Your AI Chat Assistant
Ō£ģ Answers questions, writes content & assists in coding
Ō£ģ Helps businesses with customer support
Ō£ģ Boosts learning & productivity
¤ż¢ From content to codingŌĆöChatGPT does it all!
Devin AI
¤Æ╗ Devin AI ŌĆō AI for Coders
Ō£ģ Writes, debugs & optimizes code
Ō£ģ Assists developers at all skill levels
Ō£ģ Makes coding faster & more efficient
¤æ©ŌĆŹ¤Æ╗ Let AI be your coding partner!
¤ÜĆ AI is transforming the way we work!UiPath Automation Developer Associate Training Series 2025 - Session 1



UiPath Automation Developer Associate Training Series 2025 - Session 1DianaGray10
╠²
Welcome to UiPath Automation Developer Associate Training Series 2025 - Session 1.
In this session, we will cover the following topics:
Introduction to RPA & UiPath Studio
Overview of RPA and its applications
Introduction to UiPath Studio
Variables & Data Types
Control Flows
You are requested to finish the following self-paced training for this session:
Variables, Constants and Arguments in Studio 2 modules - 1h 30m - https://academy.uipath.com/courses/variables-constants-and-arguments-in-studio
Control Flow in Studio 2 modules - 2h 15m - https:/academy.uipath.com/courses/control-flow-in-studio
Ōüē’ĖÅ For any questions you may have, please use the dedicated Forum thread. You can tag the hosts and mentors directly and they will reply as soon as possible. 2025-02-27 Tech & Play_ Fun, UX, and Community.pdf



2025-02-27 Tech & Play_ Fun, UX, and Community.pdfkatalinjordans1
╠²
2025-02-27 Tech & Play_ Fun, UX, and Community.pdfCaching for Performance Masterclass: Caching Strategies



Caching for Performance Masterclass: Caching StrategiesScyllaDB
╠²
Exploring the tradeoffs of common caching strategies ŌĆō and a look at the architectural differences.
- Which strategies exist
- When to apply different strategies
- ScyllaDB cache designWilliam Maclyn Murphy McRae - A Seasoned Professional Renowned



William Maclyn Murphy McRae - A Seasoned Professional RenownedWilliam Maclyn Murphy McRae
╠²
William Maclyn Murphy McRae, a logistics expert with 9+ years of experience, is known for optimizing supply chain operations and consistently exceeding industry standards. His strategic approach, combined with hands-on execution, has streamlined distribution processes, reduced lead times, and consistently delivered exceptional results.What is Blockchain and How Can Blockchain Consulting Help Businesses.pdf



What is Blockchain and How Can Blockchain Consulting Help Businesses.pdf Yodaplus Technologies Private Limited
╠²
This is a comprehensive guide explaining how blockchain technology works, its key features, and real-world applications in industries like finance, supply chain, and retail. Learn about different blockchain networks (public, private, and consortium) and the challenges businesses face in adopting blockchain. Discover how blockchain consulting can help businesses implement secure, transparent, and efficient solutions, reducing risks and optimizing operations. This guide is ideal for businesses exploring blockchain adoption and seeking expert guidance.THE BIG TEN BIOPHARMACEUTICAL MNCs: GLOBAL CAPABILITY CENTERS IN INDIA



THE BIG TEN BIOPHARMACEUTICAL MNCs: GLOBAL CAPABILITY CENTERS IN INDIASrivaanchi Nathan
╠²
This business intelligence report, "The Big Ten Biopharmaceutical MNCs: Global Capability Centers in India", provides an in-depth analysis of the operations and contributions of the Global Capability Centers (GCCs) of ten leading biopharmaceutical multinational corporations in India. The report covers AstraZeneca, Bayer, Bristol Myers Squibb, GlaxoSmithKline (GSK), Novartis, Sanofi, Roche, Pfizer, Novo Nordisk, and Eli Lilly. In this report each company's GCC is profiled with details on location, workforce size, investment, and the strategic roles these centers play in global business operations, research and development, and information technology and digital innovation.Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...



Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...Earley Information Science
╠²
What is Blockchain and How Can Blockchain Consulting Help Businesses.pdf



What is Blockchain and How Can Blockchain Consulting Help Businesses.pdf Yodaplus Technologies Private Limited
╠²
10/12 Review: What is atomic radius?
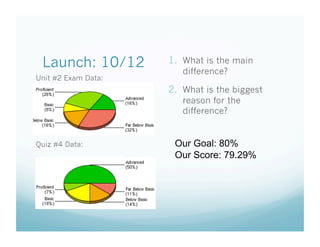
- 1. Launch: 10/12 1.ŌĆ» What is the main difference? Unit #2 Exam Data: 2.ŌĆ» What is the biggest reason for the difference? Quiz #4 Data: Our Goal: 80% Our Score: 79.29%
- 2. Launch: 10/12 1.ŌĆ» What is the main Unit #2 Exam Data: difference? 2.ŌĆ» What is the biggest reason for the difference? Quiz #4 Data: Our Goal: 80% Our Score: 79.29%
- 3. Launch: 10/12 1.ŌĆ» What is the main difference? Unit #2 Exam Data: 2.ŌĆ» What is the biggest reason for the difference? Quiz # 4 Data: Our Goal: 80% Our Score: 79.17%
- 4. Launch: 10/12 1.ŌĆ» What is the main difference? Unit #2 Exam Data: 2.ŌĆ» What is the biggest reason for the difference? Quiz # 4 Data: Our Goal: 80% Our Score: 79.00%
- 5. Mid-Term Exam Info ’éŚŌĆ» Cumulative exam ’éŚŌĆ» Unit #1: Atomic Structure ’éŚŌĆ» Unit #2: The Periodic Table ’éŚŌĆ» Unit #3: Nuclear Processes ’éŚŌĆ» Opportunity to change your grade ’éŚŌĆ» We donŌĆÖt have much time! ’éŚŌĆ» If you donŌĆÖt understand something, ask! ’éŚŌĆ» No copying
- 6. What is the trend in atomic radius? Mr. Heffner 10/12/09
- 7. Review: Period vs. Group ’éŚŌĆ» Period = row (left to right) ’éŚŌĆ» Group = column (top to bottom)
- 8. Atomic Radius ’éŚŌĆ» Atomic radius isŌĆ” ’éŚŌĆ» the size of an atom. 0 0 0 0 ’éŚŌĆ» big radius = big atom
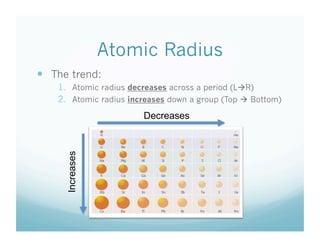
- 9. Atomic Radius ’éŚŌĆ» The trend: 1.ŌĆ» Atomic radius decreases across a period (L’āĀR) 2.ŌĆ» Atomic radius increases down a group (Top ’āĀ Bottom) Decreases Increases
- 10. Example Which has a smaller atomic radius, fluorine (F) or nitrogen (N)? ’éŚŌĆ» Step #1: Find the elements
- 11. Example Which has a smaller atomic radius, fluorine (F) or nitrogen (N)? ’éŚŌĆ» Step #2: Look at the keyword Which has a smaller atomic radius, fluorine (F) or nitrogen (N)?
- 12. Example Which has a smaller atomic radius, fluorine (F) or nitrogen (N)? ’éŚŌĆ» Step #3: Identify which is closest to He
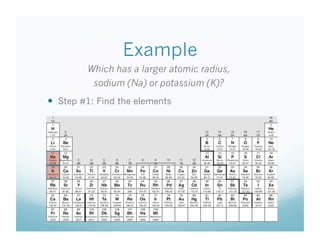
- 13. Example Which has a larger atomic radius, sodium (Na) or potassium (K)? ’éŚŌĆ» Step #1: Find the elements
- 14. Example Which has a larger atomic radius, sodium (Na) or potassium (K)? ’éŚŌĆ» Step #2: Look at the keyword Which has a larger atomic radius, sodium (Na) or potassium (K)?
- 15. Example Which has a larger atomic radius, sodium (Na) or potassium (K)? ’éŚŌĆ» Step #3: Identify which is closest to Fr
- 16. Practice Questions ’éŚŌĆ» Review Worksheet #1 ’éŚŌĆ» Use the 3-step method!
- 17. Atomic Radius ’éŚŌĆ» The trend: 1.ŌĆ» Atomic radius decreases across a period (L’āĀR) 2.ŌĆ» Atomic radius increases down a group (Top ’āĀ Bottom) Decreases Increases
- 18. Homework ’éŚŌĆ» Review your notes ’éŚŌĆ» Finish Review Worksheet #1 ’éŚŌĆ» Make a problem from scratch like question 6 or 7 on the worksheet. Solve it!



