10206462.ppt
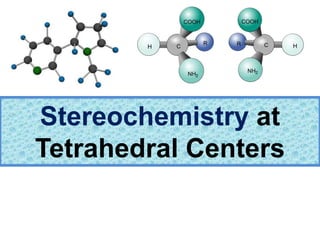
- 2. Chapter 5 2 ? Isomerism: Constitutional Isomers and Stereoisomers ©C Stereoisomers are isomers with the same molecular formula and same connectivity of atoms but different arrangement of atoms in space
- 5. Latihan
- 6. INTRODUCTION
- 7. INTRODUCTION
- 8. INTRODUCTION ? Molecules that are not identical to their mirror images are kinds of stereoisomers called enantiomers (Greek enantio, meaning Ī░oppositeĪ▒). ? It result whenever a tetrahedral carbon is bonded to four different substituents
- 9. INTRODUCTION
- 10. CHIRALITY ? A molecule that is not identical to its mirror image is said to be chiral (ky-ral, from the Greek cheir, meaning Ī░handĪ▒) ? A molecule is chiral if it has no plane of symmetry ? A plane of symmetry : a plane that cuts through the middle of a molecule (or any object) in such a way that one half of the molecule or object is a mirror image of the other half.
- 11. CHIRALITY ? Chirality centers = stereocenter, asymmetric center, and stereogenic center
- 12. CHIRALITY
- 13. EXERCISE 1 ? Which of the following objects are chiral? Achiral Achiral Chiral Chiral
- 14. EXERCISE 2 ? Identify the chirality center(s) in each * * * *
- 15. Optical Activity ? The study of chirality : Jean-Baptiste Biot
- 16. Optical Activity ? Organic substances that rotate the plane of polarization through an angle (”┴): optically active ? Optically active molecules rotate polarized light to the left (counterclockwise): levorotatory ? minus sign (-) ? Optically active molecules rotate polarized light to the right (clockwise) : dextrorotatory ? plus sign (+)
- 17. Optical Activity ? The specific rotation [”┴]D : the observed rotation when light of 589.6 nanometer (nm; 1 nm = 10-9 m) wavelength is used with a sample pathlength (l) of 1 decimeter (dm; 1 dm = 10 cm) and a sample concentration (c) of 1 g/cm3
- 18. Optical Activity
- 19. EXERCISE 3 ? a 1.20 g sample of cocaine, [”┴]D = -16, was dissolved in 7.50 mL of chloroform and placed in a sample tube having a pathlength of 5.00 cm. What was the observed rotation?
- 20. Sequence Rules for Specifying Configuration Rule 1 ? Look at the four atoms directly attached to the chirality center, and rank them according to atomic number
- 21. Sequence Rules for Specifying Configuration Rule 2 ? If a decision canĪ»t be reached by ranking the first atoms in the substituent, look at the second, third, or fourth atoms away from the chirality center until the first difference is found
- 22. Sequence Rules for Specifying Configuration Rule 3 ? Multiple-bonded atoms are equivalent to the same number of single bonded atoms.
- 23. Sequence Rules for Specifying Configuration ? the group with the lowest ranking (4) points directly back, away from us ? (1?2?3) is clockwise: the chirality center has the R configuration (Latin rectus, meaning Ī░rightĪ▒) ? (1?2?3) is counterclockwise: the chirality center has the S configuration (Latin sinister, meaning Ī░leftĪ▒)
- 27. ? Prospective formulas (3d) ? Fischer Projections (Proyeksi Fischer) ©C Merepresentasikan molekul (senyawa) kiral dalam 2 dimensi ©C C stereogenik dihilangkan dan dinyatakan sebagai titik silang garis vertikal & horison ©C Garis vertikal menunjukkan bahwa gugus-gugus berada dibawah bidang kertas menjauhi mata ©C Garis Horisontal menunjukkan bahwa gugus berada diatas bidang kertas mendekati mata Drawing Enantiomers
- 28. ? Tentukan yang mana enansiomer R dan S dari 2-bromobutana ? jawaban: R S
- 29. EXERCISE 4 ? Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration
- 30. EXERCISE 5 ? Draw a tetrahedral representation and fisher projection of (R)-2-chlorobutane
- 31. ©C Apakah senyawa A dan B merupakan senyawa identik atau enansiomer? ? Manipulasi B untuk melihat apakah dapat Ī░superimposĪ▒ dengan A ? Exchange 2 groups to try to convert B into A ©C One exchange of groups leads to the enantiomer of B ©C Two exchanges of groups leads back to B
- 32. Diastereomers ? a molecule with n chirality centers can have up to 2n stereoisomers ? ?
- 33. Diastereomers ? Diastereomers : stereoisomers that are not mirror images The difference between enantiomers and diastereomers: ? Enantiomers have opposite configurations at all chirality centers ? Diastereomers have opposite configurations at some (one or more) chirality centers but the same configuration at others.
- 34. Meso Compounds
- 35. Meso Compounds ? The 2R,3S and 2S,3R structures are identical because the molecule has a plane of symmetry ? achiral ? Compounds that are achiral, yet contain chirality centers, are called meso compounds
- 36. Meso Compounds
- 37. Racemic Mixtures ? a racemate or racemic mixture : a 50:50 mixture of the two chiral enantiomers ? denoted by either the symbol (+) or the prefix d,l ? Racemates show no optical rotation
- 38. Prochirality ? Prochiral : a molecule that can be converted from achiral to chiral in a single chemical step ? Compounds with tetrahedral, sp3-hybridized atoms can also be prochiral
- 39. Chiral Drugs ? Dugs that come from natural sources, either directly or after chemical modification, are usually chiral and are generally found only as a single enantiomer rather than as a racemate ? Penicillin V, an antibiotic isolated from the Penicillium mold, has the 2S,5R,6R configuration. Its enantiomer, which does not occur naturally but can be made in the laboratory, has no antibiotic activity.
- 40. Chiral Drugs ? Drugs that are made entirely in the laboratory either are achiral or, if chiral, are often produced and sold as racemates. ? Ibuprofen, has one chirality center and is sold commercially under such trade names as Advil, Nuprin, and Motrin as a 50;50 mixture of R and S. ? The S enantiomer is active as an analgesic and anti-inflammatory agent. The R enantiomer of ibuprofen is inactive, although it is slowly converted in the body to the active S form.
- 41. THANK YOU















![Optical Activity
? The specific rotation [”┴]D : the observed rotation
when light of 589.6 nanometer (nm; 1 nm = 10-9 m)
wavelength is used with a sample pathlength (l) of 1
decimeter (dm; 1 dm = 10 cm) and a sample
concentration (c) of 1 g/cm3](https://image.slidesharecdn.com/10206462-221121123704-e3344d05/85/10206462-ppt-17-320.jpg)

![EXERCISE 3
? a 1.20 g sample of cocaine, [”┴]D = -16, was dissolved in
7.50 mL of chloroform and placed in a sample tube
having a pathlength of 5.00 cm. What was the observed
rotation?](https://image.slidesharecdn.com/10206462-221121123704-e3344d05/85/10206462-ppt-19-320.jpg)





















