11/4 What are Lewis structures? - Part II
- 1. Launch: 11/4   Grab your binder and immediately take a seat!   Place homework (practice questions) on desk 1.  Draw the correct Lewis structures for the following atoms. Be sure to use the 3-steps! i.  Br2 ii.  H2S iii.  CCl4
- 2. Schedule   We have an exam in 6 days!   We don’t have time to waste!   Follow our 7 class rules   You may not use inappropriate language   If you have a question, ask!
- 3. What are Lewis structures? – Part II Mr. Heffner 11/4/09
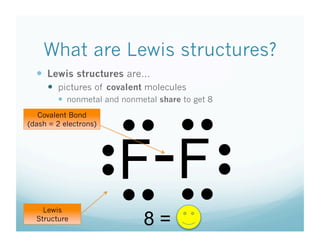
- 4. What are Lewis structures?   Lewis structures are…   pictures of covalent molecules   nonmetal and nonmetal share to get 8 • •  • •  Covalent Bond F- F (dash = 2 electrons) • •  • •  Lewis Structure • • = • •  8
- 5. What are Lewis structures?   Cocaine Double Bond   C17H21NO4 2 dashes = 4 electrons
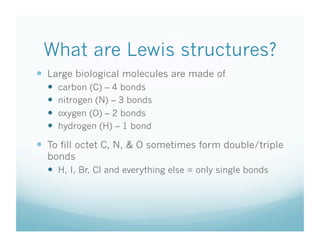
- 6. What are Lewis structures?   Large biological molecules are made of   carbon (C) – 4 bonds   nitrogen (N) – 3 bonds   oxygen (O) – 2 bonds   hydrogen (H) – 1 bond   To fill octet C, N, & O sometimes form double/triple bonds   H, I, Br, Cl and everything else = only single bonds
- 7. What is a Lewis structure?   There is a 3-step process for creating Lewis structures: 1.  Draw Lewis symbols & count dots 2.  Arrange atoms so they have a full octet   If necessary form a double/triple bond 3.  Count dots & make bonds
- 8. Example Draw the Lewis structure for O2   Step 1: Draw Lewis symbols & count dots • •  • •  O O • •  •  • •  •  •  •  12 dots
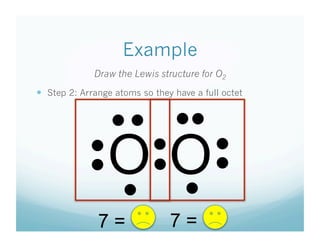
- 9. Example Draw the Lewis structure for O2   Step 2: Arrange atoms so they have a full octet • •  • •  O O • •  •  • •  •  •  7= •  7=
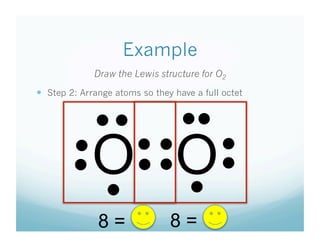
- 10. Example Draw the Lewis structure for O2   Step 2: Arrange atoms so they have a full octet • •  • •  •  O O•  • •  •  • •  •  •  •  8 = 8=
- 11. Example Draw the Lewis structure for O2   Step 3: Count dots & make bonds • •  • •  •  O O•  • •  •  • •  •  12 dots Double Bond 2 dashes = 4 electrons
- 12. Example Draw the Lewis structure for N2
- 13. Whiteboards   Work in pairs   Trade-off marker every question   You have 60 seconds to draw the answer   Show all three steps!   Lift board only when prompted
- 14. CCl4
- 15. CO2
- 16. CH2Cl2
- 17. N2
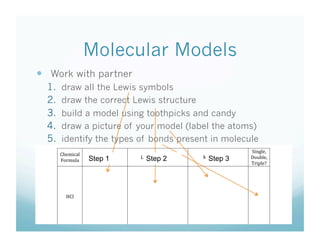
- 18. Molecular Models   Work with partner 1.  draw all the Lewis symbols 2.  draw the correct Lewis structure 3. !"#$%&''(&"$ a model using toothpicks and)*+$%,-.,"/$ build $ candy 4. 01&2345"-$ a picture of your model (label the 6678$ draw $ atoms) *",9539&$:;&453<(4=$$!<>&9;>,"$!</&>4$ 5.  identify the types of bonds present in molecule $ +3(A>&B$ 01&239,>$ )&.34$+-2@<>4$ Step 1 )&.34$+5";95;"&$ Step 2 !</&>$*395;"&$ Step 3 C<;@>&B$ ?<"2;>,$ D"3E>&F$ $ %0>$ $ $ $ $
- 19. Exit Slip 1.  The bonds in water (H2O) are a.  single bonds b.  ionic bonds c.  triple bonds d.  double bonds
- 20. Exit Slip 2.  The correct Lewis structure for CO2 is
- 21. Exit Slip 3.  Which of the following molecules contains a covalent triple bond? a.  C2H2 b.  I2 c.  NH3 d.  N2
- 22. Exit Slip 4.  Bubble in A Then, draw the Lewis structure for SiF4
- 23. Exit Slip 5.  Bubble in A Then, draw the Lewis symbol for O2
- 24. Homework   Finish Lewis symbol and ions worksheet