2) study of utilization of radioactive isotopes in the investigation of biogenetic studies
- 1. Study of utilization of Radioactive Isotopes in the Investigation of Biogenetic studies Presented By Sonali Gadge P R Patil Institute of Pharmacy, Talegaon (SP), Dist-Wardha.
- 2. Isotopes- Iso= Same (Equal), Topes=Place ŌĆó They occupy same place in periodic table. ŌĆ£Elements with same atomic number but different atomic weight (Same number of protons but differ in neutrons). ŌĆó Example- 12C6 13C6 14C6 (Isotopes) Atomic mass = No. of Protons+ No. of Neutrons Atomic Number = No. of protons Hydrogen Isotopes: 1H1 2H1 3H1 [1P] [1P, 1N] [1P, 2 N] Hydrogen Deuterium Tritium
- 3. Two types of Isotopes 1. Radioactive Isotopes (Radioisotopes)- Radio (Radiation ) + Isotopes Unstable Isotopes The isotopes which emits the radiation are called Radioisotopes. Decay with the emission of radiation (╬▒, ╬▓, ╬│ radiation). Example - 3H, 14C, 35S, 131I, 24Na, 42K, 35S, 35P, 131I - For biological Investigation- Carbon and Hydrogen - For Metabolic studies- S, P and alkali and alkaline earth metals are used. - For studies on proteins, alkaloids and amino acids-labelled nitrogen atom give more specific information. - 3H compound is commercially available. 2. Stable Isotopes: ŌĆó Stable isotopes are non-radioactive forms of atoms (they do not emit radiations). ŌĆó Although they do not emit radiations, their unique properties enable them to be used in a broad variety of applications, including water and soil management, environmental studies, nutrition assessment studies and forensics. Examples- 2H, 13C, 15N, 18O - Used for labelled compounds as possible intermediates in biosynthetic pathways - Usual method of detection are: - MASS Spectroscopy [15N, 18O] - NMR Spectroscopy [2H, 13Cl]
- 4. Radiolabelled Tracers (Radiolabelled compound) ŌĆó When one or more atom of chemical compound replaced by radioisotopes used- for the study of the biosynthetic pathway, is known as Radiotracers. Radiotracer Technique: The technique which utilises radioactive labelled compound to find out or to trace various precursors and intermediates involved at different stages of biosynthetic pathway at given rate and time. In this technique, different isotope, mainly the radioactive isotopes which are incorporated into the presumed precursor of plant metabolites and used as marker in the biogenic studies.
- 5. Steps in Tracer Technique 1. Selection of Radioisotopes 2. Preparation of Radioisotopes 3. Introduction/Insertion of Radiolabelled compound in biological system (Plant part) 4. Seperation and determination of labelled compound in various biochemical reaction
- 6. Preparation of labelled compound: (a) Growing chlorella in atmosphere of 14CO2 (b) Nuclear Reactor/Acceralator 14N7 + 1n0 14C6 + 1P1 (c) Tritium gas: Tritium labelled compound (3H1) are commercially available. Tritium labelling is effected by catallytic exchange in aqueous media by hydrogenation of unsaturated compound with tritium gas. (d) by the use of Organic Synthesis: CH3MgBr + 14CO2 CH3 14COOHMgBr + H2O CH3 14COOH+ MgOHBr Radiolabelled Acetic acid
- 7. Insertion of Radiolabelled compound in plant part Precaution ŌĆó The precursor should react at necessary site of synthesis in plants. ŌĆó Plant at the experiment time should synthesize the compound under investigation. ŌĆó The dose given is for short period. 1. Root feeding: When the root is biosynthetic site (Ex- Tobacco and Datura alkaloid). 2. Stem Feeding: Cut end of stem immersed in water, nutrient and radiolabelled compound. 3. Direct Injection: Which have hollow stem (Umbelliferous fruits). 4. Infilteration: It is also called Wick Feeding method. 5. Floating method: Substrate solution which contain Radioactive compound. 6. Spray technique
- 8. Separation or Isolation of Radiolabelled compound and detection of radioisotope labelled compound Depends on Nature of drugs and Sources of drugs- Soft tissue- Infusion, Maceration Hard Tissue- Decoction, hot percolation Unorganised drugs- Maceration Alkaloid, Glycoside, Flavonoids- Slightly polar solvent
- 9. Detection and assay of Radioactive labelled compound Detectors system used (Analysis of Isotopic content) (a) Geiger ŌĆōMuller Counter- Detection and measurement of all types of radiation (b) Liquid Scintillation Counter ŌĆō Scintillators are used (For ╬▓ emitting radioisotopes) (c) Gas Ionization Chamber (d) Bernstein-Bellentine Counter (e) Mass spectroscopy (f) NMR Electrodemeter (g) Autoradiography ŌĆō to trace the location of radioactive isotope in biological system (h) Radio paper chromatography
- 10. Methods in Tracer Technique 1. Precursor ŌĆō Product sequence In this technique, the presumed precursor of the constituent under investigation on a labelled form is fed into the plant and after a suitable time the constituent is isolated, purified and radioactivity is determined. Disadvantages: The radioactivity of isolated compound alone is not usually sufficient evidence that the particular compound fed is direct precursor, because substance may enter the general metabolic pathway and from there may become randomly distributed through a whole range of product.
- 11. 2. Double and Multiple Labelling This method give the evidence for nature of biochemical incorporation of precursor arises double and triple labelling. In this method specifically labelled precursor and their subsequent degradation of recover product are more employed. Application: 1. This method is extensively applied to study the biogenesis of plant secondary metabolite. 2. Used for study of morpheine alkaloid.
- 13. 3. Competitive Feeding If incorporation is obtained it is necessary to consider whether this infact, the normal route of synthesis in plant not the subsidary pathway. Competitive feeding can distinguish whether B and BŌĆÖ is the normal intermediate for the formation of C from A. Applicatiion: This method is used for the biogenesis of propane alkaloids. Biosynthesis of hemlock alkaloids (conline, conhhydrine, etc) e.g. biosynthesis of alkaloids of Conium manuculatum (Hemlock) using 14C labelled compounds.
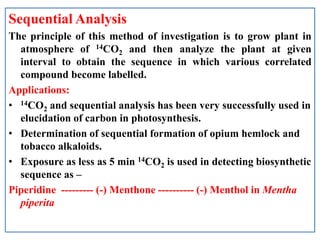
- 14. Sequential Analysis The principle of this method of investigation is to grow plant in atmosphere of 14CO2 and then analyze the plant at given interval to obtain the sequence in which various correlated compound become labelled. Applications: ŌĆó 14CO2 and sequential analysis has been very successfully used in elucidation of carbon in photosynthesis. ŌĆó Determination of sequential formation of opium hemlock and tobacco alkaloids. ŌĆó Exposure as less as 5 min 14CO2 is used in detecting biosynthetic sequence as ŌĆō Piperidine --------- (-) Menthone ---------- (-) Menthol in Mentha piperita
- 16. Applications of Tracer Technique 1. Study of squalene cyclization by use of 14C, 3H labelled mevalonic acid. 2. Interrelationship among 4-methyl sterols and 4,4-dimethyl sterols, by use of 14C acetate. 3. Terpenoid biosynthesis by chloroplast isolated in organic solvent by use of 2-14C mevalonate. 4. Study the formation of cinnamic acid in pathway of coumarin from labelled coumarin. 5. Origin of carbon and nitrogen atoms of purine ring system by use of 14C or 15N labelled precursor. 6. Study of formation of scopoletin by use of labelled phenylalanine. 7. By use of 45Ca as tracer, found that the uptake of calcium by plants from the soil (CaO and CaCO2). 8. By adding ammonium phosphate labelled with 32P of known specific activity the uptake of phosphorus is followed by measuring the radioactivity as label reaches first in lower part of the plant, than the upper part i.e. branches, leaves, etc.
![Isotopes-
Iso= Same (Equal), Topes=Place
ŌĆó They occupy same place in periodic table. ŌĆ£Elements
with same atomic number but different atomic
weight (Same number of protons but differ in
neutrons).
ŌĆó Example- 12C6
13C6
14C6 (Isotopes)
Atomic mass = No. of Protons+ No. of Neutrons
Atomic Number = No. of protons
Hydrogen Isotopes:
1H1
2H1
3H1
[1P] [1P, 1N] [1P, 2 N]
Hydrogen Deuterium Tritium](https://image.slidesharecdn.com/2studyofutilizationofradioactiveisotopesintheinvestigationofbiogeneticstudies-200703114639/85/2-study-of-utilization-of-radioactive-isotopes-in-the-investigation-of-biogenetic-studies-2-320.jpg)
![Two types of Isotopes
1. Radioactive Isotopes (Radioisotopes)- Radio (Radiation ) + Isotopes
Unstable Isotopes
The isotopes which emits the radiation are called Radioisotopes.
Decay with the emission of radiation (╬▒, ╬▓, ╬│ radiation).
Example - 3H, 14C, 35S, 131I, 24Na, 42K, 35S, 35P, 131I
- For biological Investigation- Carbon and Hydrogen
- For Metabolic studies- S, P and alkali and alkaline earth metals are used.
- For studies on proteins, alkaloids and amino acids-labelled nitrogen atom give
more specific information.
- 3H compound is commercially available.
2. Stable Isotopes:
ŌĆó Stable isotopes are non-radioactive forms of atoms (they do not emit
radiations).
ŌĆó Although they do not emit radiations, their unique properties enable them to
be used in a broad variety of applications, including water and soil
management, environmental studies, nutrition assessment studies and
forensics.
Examples- 2H, 13C, 15N, 18O
- Used for labelled compounds as possible intermediates in biosynthetic
pathways
- Usual method of detection are: - MASS Spectroscopy [15N, 18O]
- NMR Spectroscopy [2H, 13Cl]](https://image.slidesharecdn.com/2studyofutilizationofradioactiveisotopesintheinvestigationofbiogeneticstudies-200703114639/85/2-study-of-utilization-of-radioactive-isotopes-in-the-investigation-of-biogenetic-studies-3-320.jpg)