Indwelling Urinary Catheter -適応について真剣に考える-
- 1. Indwelling Urinary Catheter 東京慈恵会医科?学葛飾医療センター?酔部 福島東浩 1 2021年12?14?慈恵医?ICU勉強会 適 応 に つ い て 真 剣 に 考 え る
- 2. 今回のテーマ ? はじめに ? abbreviation ? 歴史 ? 尿道カテーテル留置の現状 ? ガイドライン上の適応 ? 尿量モニタリング ? AKIの診断 ? 循環評価 ? 尿排出 ? ?分バランスの調整 ? 術後尿閉 2 ? 合併症 ? CA-UTI ? ?感染性合併症 ? 代替?法 ? 超?波診断装置 ? 体重測定 ? 尿道カテーテル留置は減らせるか? ? 最後に
- 3. はじめに 3
- 4. Abbreviations AHRQ: Agency for Healthcare Research and Quality AKI: Acute Kidney Injury BS: Bladder Scanner CAH: Critical-access hospital CAUTI: Catheter Associated Urinary Tract Infection CDC: Centers for Disease Control and Prevention HICPAC: Health Infection Control Practice Advisory Committee ISC: Intermittent Straight Catheter 4 IUC: Indwelling Urinary Catheter POUR: Postoperative urinary retention QI: Quality Improvement SCr: serum creatinine UO: Urinary Output US: Ultrasound UTI: Urinary Tract Infection UVol: Urine Volume
- 5. Indwelling Urinary (Urethral) Catheter ?本語訳は? ○○カテーテル留置 ○○留置カテーテル ○○ à 尿路、尿道、膀胱 「尿カテ」と省略していることも多々あります 表現に?定の決まりがなく、所々変わることをご了承下さい 5
- 6. 歴史 6 Feneley RC et al. J Med Eng Technol. 2015;39(8):459-70. Erratum in: J Med Eng Technol. 2016;40(2):59. PMID: 26383168 ? 1500BC エジプト パピルスに銅管, 葦, 藁, 巻いた椰?の葉での尿閉治療の記述 ? 400BC ヒポクラテスが鉛の管を使? ? 18世紀頃からゴム製のカテーテルが開発 ? 1933 Frederic Foley博?の開発した尿道カテーテルがBARD社から発売
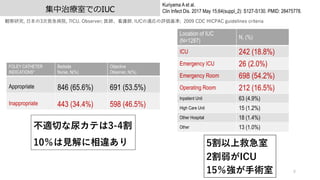
- 8. 集中治療室でのIUC 8 Kuriyama A et al. Clin Infect Dis. 2017 May 15;64(suppl_2): S127-S130. PMID: 28475778. 観察研究, ?本の3次救急病院, 7ICU. Observer; 医師、看護師. IUCの適応の評価基準; 2009 CDC HICPAC guidelines criteria FOLEY CATHETER INDICATIONS* Bedside Nurse, N(%) Objective Observer, N(%) Appropriate 846 (65.6%) 691 (53.5%) Inappropriate 443 (34.4%) 598 (46.5%) Location of IUC (N=1287) N, (%) ICU 242 (18.8%) Emergency ICU 26 (2.0%) Emergency Room 698 (54.2%) Operating Room 212 (16.5%) Inpatient Unit 63 (4.9%) High Care Unit 15 (1.2%) Other Hospital 18 (1.4%) Other 13 (1.0%) 不適切な尿カテは3-4割 10%は?解に相違あり 5割以上救急室 2割弱がICU 15%強が?術室
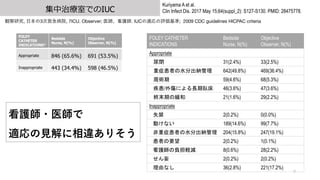
- 9. 集中治療室でのIUC 9 Kuriyama A et al. Clin Infect Dis. 2017 May 15;64(suppl_2): S127-S130. PMID: 28475778. 観察研究, ?本の3次救急病院, 7ICU. Observer; 医師、看護師. IUCの適応の評価基準; 2009 CDC guidelines HICPAC criteria FOLEY CATHETER INDICATIONS* Bedside Nurse, N(%) Objective Observer, N(%) Appropriate 846 (65.6%) 691 (53.5%) Inappropriate 443 (34.4%) 598 (46.5%) FOLEY CATHETER INDICATIONS Bedside Nurse, N(%) Objective Observer, N(%) Appropriate 尿閉 31(2.4%) 33(2.5%) 重症患者の水分出納管理 642(49.8%) 469(36.4%) 周術期 59(4.6%) 68(5.3%) 疾患/外傷による長期臥床 46(3.6%) 47(3.6%) 終末期の緩和 21(1.6%) 29(2.2%) Inappropriate 失禁 2(0.2%) 0(0.0%) 動けない 189(14.6%) 99(7.7%) 非重症患者の水分出納管理 204(15.8%) 247(19.1%) 患者の要望 2(0.2%) 1(0.1%) 看護師の負担軽減 8(0.6%) 28(2.2%) せん妄 2(0.2%) 2(0.2%) 理由なし 36(2.8%) 221(17.2%) 看護師?医師で 適応の?解に相違ありそう
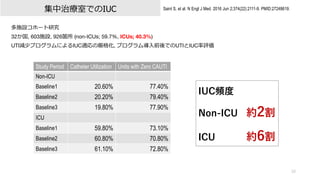
- 10. 10 Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619. 多施設コホート研究 32か国, 603施設, 926箇所 (non-ICUs; 59.7%, ICUs; 40.3%) UTI減少プログラムによるIUC適応の厳格化, プログラム導?前後でのUTIとIUC率評価 集中治療室でのIUC Study Period Catheter Utilization Units with Zero CAUTI Non-ICU Baseline1 20.60% 77.40% Baseline2 20.20% 79.40% Baseline3 19.80% 77.90% ICU Baseline1 59.80% 73.10% Baseline2 60.80% 70.80% Baseline3 61.10% 72.80% IUC頻度 Non-ICU 約2割 ICU 約6割
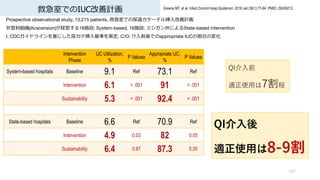
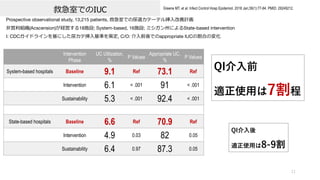
- 11. 救急室でのIUC 11 Greene MT, et al. Infect Control Hosp Epidemiol. 2018 Jan;39(1):77-84. PMID: 29249212. Prospective observational study, 13,215 patients, 救急室での尿道カテーテル挿?改善計画 ?営利組織(Acscension)が経営する18施設; System-based, 16施設; ミシガン州によるState-based intervention I: CDCガイドラインを基にした尿カテ挿?基準を策定, C/O: 介?前後でのappropriate IUCの割合の変化 Intervention Phase UC Utilization, % P Valuea Appropriate UC, % P Valuea System-based hospitals Baseline 9.1 Ref 73.1 Ref Intervention 6.1 < .001 91 < .001 Sustainability 5.3 < .001 92.4 < .001 State-based hospitals Baseline 6.6 Ref 70.9 Ref Intervention 4.9 0.03 82 0.05 Sustainability 6.4 0.97 87.3 0.05 QI介?前 適正使?は7割程 QI介?後 適正使?は8-9割
- 12. 救急室でのIUC 12 Fakih MG, et al. Acad Emerg Med. 2010 Mar;17(3):337-40. PMID: 20370769.. 救急室での尿道カテーテル減少プロジェクトを検証 before-after, single center, 769 tertiatry care hospital, 2517 patients Compliant with guidelines, n = 203 (63.0%) N (%) Non?intensive care 6 L /min oxygen 40 (12.4) Output monitoring in intensive care 39 (12.1) Emergent pelvic ultrasound 33 (10.2) Intubated 32 (9.9) Neurogenic bladder 14 (4.3) Emergency surgery 12 (3.7) Urinary obstruction 10 (3.1) Unresponsive 7 (2.2) Acute hip fracture 6 (1.9) Urologic procedures 4 (1.2) Acute mental status changes with agitation 4 (1.2) Stage 3 or 4 sacral decubitus ulcers with incontinence 1 (0.3) Hospice or palliative care 1 (0.3) Noncompliant with guidelines, n = 119 (37.0%) No clear reason 64 (19.9) Oxygen supplementation <6 L ? min 26 (8.1) Dementia 16 (5.0) Urine specimen collection 5 (1.6) Incontinence 3 (0.9) Patient request 3 (0.9) Output monitoring outside intensive care 2 (0.6) ガイドライン非遵守IUCの理由 理由なしが約20%
- 15. Guideline for prevention of catheter-associated urinary tract infections 2009 15 https://www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines-H.pdf 1981年CDCよりUTI予防についてのガイドラインが発効 2009年に改訂 2017年に?部修正
- 16. Guideline for prevention of catheter-associated urinary tract infections 2009 16 https://www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines-H.pdf https://www.info-cdcwatch.jp/views/pdf/CDC_guideline2009.pdf A. 尿道留置カテーテルの適切な使?例 患者に急性の尿閉または膀胱出?部閉塞がある 重篤な患者の尿量の正確な測定が必要である 特定の外科?技のための周術期使? ● 泌尿?殖器の周辺構造で泌尿器科?術または他の?術を受ける患者 ● ?時間の?術が予測される患者(このために挿?されるカテーテルは?酔後回復室(PACU:post-anesthesia care unit)で抜去する) ● 術中に?量の点滴または利尿剤が投与されることが予測される患者 ● 尿量の術中モニタリングが必要な患者 尿失禁患者の仙椎部または会陰部にある開放創の治癒を促すため 患者を?期に固定する必要がある(例:胸椎または腰椎が潜在的に不安定、?盤?折のような多発外傷) 必要に応じて終末期ケアの快適さを改善するため B. 尿道留置カテーテルの不適切な使?例 尿失禁のある患者または居住者の看護ケアの代わりとしての使? 患者が?発排尿をできるときに、培養その他の診断検査のために採尿する?段としての使? 適切な適応が認められない場合の術後?期間の使?(例:尿道または周辺構造の修復、硬膜外?酔の作?遷延など)
- 17. 17 AHRQ Safety Program for Reducing CAUTI in Hospitals https://www.ahrq.gov/hai/cauti-tools/impl-guide/index.htmL
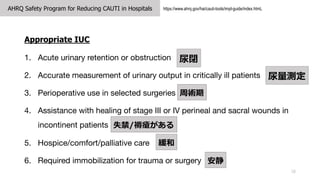
- 18. AHRQ Safety Program for Reducing CAUTI in Hospitals 18 https://www.ahrq.gov/hai/cauti-tools/impl-guide/index.htmL Appropriate IUC 1. Acute urinary retention or obstruction 2. Accurate measurement of urinary output in critically ill patients 3. Perioperative use in selected surgeries 4. Assistance with healing of stage III or IV perineal and sacral wounds in incontinent patients 5. Hospice/comfort/palliative care 6. Required immobilization for trauma or surgery 尿閉 尿量測定 周術期 失禁/褥瘡がある 緩和 安静
- 19. 19 Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619. Inappropriate IUC 1. Urine output monitoring that can be obtained by means other than an indwelling urinary catheter 2. Incontinence without a sacral or perineal pressure sore 3. Prolonged postoperative use 4. Other potentially inappropriate uses of urinary catheters include the following: ① Patients who are being transferred within or from an acute care facility ② Morbid obesity or immobility ③ Confusion or dementia ④ Patient and or family request AHRQ Safety Program for Reducing CAUTI in Hospitals 替わりがある 失禁/褥瘡がない 術後?期 搬送時 肥満などで動けないだけ せん妄/認知機能低下 要望
- 20. 救急室でのIUC 20 https://viaaerearcp.files.wordpress.com/2018/02/atls-2018.pdf Advanced Trauma Life Support ATLS 10th edition Initial Assessment and Management Urinary Catheter Urinary output is a sensitive indicator of the patient’s volume status and reflects renal perfusion. Monitoring of urinary output is best accomplished by insertion of an indwelling bladder catheter. In addition, a urine specimen should be submitted for routine laboratory analysis. Transurethral bladder catheterization is contraindicated for patients who may have urethral injury. Suspect a urethral injury in the presence of either blood at the urethral meatus or perineal ecchymosis. Accordingly, do not insert a urinary catheter before examining the perineum and genitalia. When urethral injury is suspected, confirm urethral integrity by performing a retrograde urethrogram before the catheter is inserted. At times anatomic abnormalities (e.g., urethral stricture or prostatic hypertrophy) preclude placement of indwelling bladder catheters, despite appropriate technique. Nonspecialists should avoid excessive manipulation of the urethra and the use of specialized instrumentation. Consult a urologist early. 尿量モニタリングは尿道カテーテル留置が最も適切
- 21. 救急室でのIUC 21 https://www.ahrq.gov/hai/cauti-tools/archived-webinars/ed-catheter-insertions-slides.htmL アメリカ医療研究品質機構 Agency for Healthcare Research and Quality 救急室での不適切な尿カテについて?及
- 22. 数値目標や対象が不明確 22
- 23. 適応を再検証しましょう 23
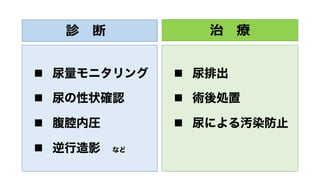
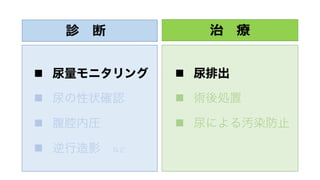
- 24. 診 断 n 尿量モニタリング n 尿の性状確認 n 腹腔内圧 n 逆行造影 など 治 療 n 尿排出 n 術後処置 n 尿による汚染防止
- 25. 診 断 n 尿量モニタリング n 尿の性状確認 n 腹腔内圧 n 逆行造影 など 治 療 n 尿排出 n 術後処置 n 尿による汚染防止
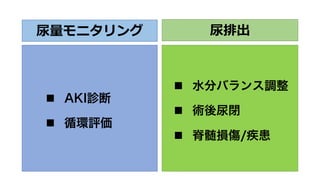
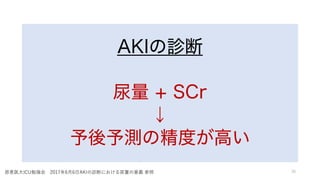
- 26. 尿量モニタリング n AKI診断 n 循環評価 尿排出 n 水分バランス調整 n 術後尿閉 n 脊髄損傷/疾患
- 27. 尿量モニタリング n AKI診断 n 循環評価 尿排出 n 水分バランス調整 n 術後尿閉 n 脊髄損傷/疾患
- 28. 尿量モニタリング n AKI診断 n 循環評価 尿排出 n 水分バランス調整 n 術後尿閉 n 脊髄損傷/疾患
- 29. 救急外傷患者でのIUC ?盤外傷時のIUC 出?性ショックで尿量モニタリングが必要になる 膀胱内への??注? à 圧迫?? ?盤外傷の約20%尿路損傷合併といわれる ATLS 10th edition When urethral injury is suspected, confirm urethral integrity by performing a retrograde urethrogram before the catheter is inserted. 衝動損傷が疑われる場合は尿カテ挿?前に逆?尿路造影 ?盤外傷の話になってくるので今回は割愛 29 ?Coccolini F et al. World J Emerg Surg. 2019 Dec 2;14:54. PMID: 31827593. ?https://viaaerearcp.files.wordpress.com/2018/02/atls-2018.pdf
- 30. 尿量モニタリング 30
- 31. AKIの診断 循環評価 31
- 32. AKIの診断 循環評価 32
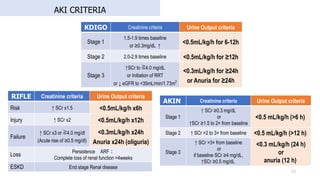
- 33. AKI CRITERIA 33 RIFLE Creatinine criteria Urine Output criteria Risk ↑ SCr x1.5 <0.5mL/kg/h x6h Injury ↑ SCr x2 <0.5mL/kg/h x12h Failure ↑ SCr x3 or ≧4.0 mg/dl (Acute rise of ≥0.5 mg/dl) <0.3mL/kg/h x24h Anuria x24h (oliguria) Loss Persistence ARF? Complete loss of renal function >4weeks ESKD End stage Renal disease KDIGO Creatinine criteria Urine Output criteria Stage 1 1.5-1.9 times baseline or ≥0.3mg/dL ↑ <0.5mL/kg/h for 6-12h Stage 2 2.0-2.9 times baseline <0.5mL/kg/h for ≥12h Stage 3 ↑SCr to ≧4.0 mg/dL or Initiation of RRT or ↓ eGFR to <35mL/min/1.73m2 <0.3mL/kg/h for ≥24h or Anuria for ≥24h AKIN Creatinine criteria Urine Output criteria Stage 1 ↑ SCr ≥0.3 mg/dL or ↑SCr ≥1.5 to 2× from baseline <0.5 mL/kg/h (>6 h) Stage 2 ↑ SCr >2 to 3× from baseline <0.5 mL/kg/h (>12 h) Stage 3 ↑ SCr >3× from baseline or if baseline SCr ≥4 mg/dL, ↑SCr ≥0.5 mg/dL <0.3 mL/kg/h (24 h) or anuria (12 h)
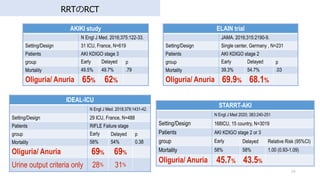
- 34. AKIKI study N Engl J Med. 2016;375:122-33. Setting/Design 31 ICU, France, N=619 Patients AKI KDIGO stage 3 group Early Delayed p Mortality 49.5% 49.7% .79 Oliguria/ Anuria 65% 62% 34 RRTのRCT ELAIN trial JAMA. 2016;315:2190-9. Setting/Design Single center, Germany , N=231 Patients AKI KDIGO stage 2 group Early Delayed p Mortality 39.3% 54.7% .03 Oliguria/ Anuria 69.9% 68.1% IDEAL-ICU N Engl J Med. 2018;379:1431-42. Setting/Design 29 ICU, France, N=488 Patients RIFLE Failure stage group Early Delayed p Mortality 58% 54% 0.38 Oliguria/ Anuria 69% 69% Urine output criteria only 28% 31% STARRT-AKI N Engl J Med 2020; 383:240-251 Setting/Design 168ICU, 15 country, N=3019 Patients AKI KDIGO stage 2 or 3 group Early Delayed Relative Risk (95%CI) Mortality 58% 58% 1.00 (0.93-1.09) Oliguria/ Anuria 45.7% 43.5%
- 35. 尿量モニタリングと死亡リスク 35 Jin K, et al. Chest. 2017 Nov;152(5):972-979. PMID: 28527880. ICUデータベースを?いて尿量モニタリング厳密さとAKI発症予後の関連を評価 , 8 ICU, retrospective cohort study 15,724 adults, AKI diagnosis; KDIGO criteria UO Intensive monitoring 死亡リスクが低かった Intensive monitoring: UO intensive monitoring, hourly recordings; no gaps of > 3 hours for the initial 48 hours SC intensive monitoring, 3 calendar days of SC data Less Intensive monitoring: not meeting intensive monitoring criteria in the 7 days Intensive Monitoring by UO SC Only UO Only Both SC and UO AKI 166 (4.1%) 1,795 (44.3%) 568 (14%) No AKI 1,520 (37.5%) TOTAL 4,049 Non-intensive Monitoring by UO SC Only UO Only Both SC and UO AKI 693 (5.9%) 4,432(38%) 2337(20%) No AKI 4,214 (36.1%) TOTAL 11,675 Outcome: 30-Day Mortality HR (95% CI) P Value Model 1: UO Monitoringa AKI Monitoring–/AKI+ Reference Monitoring+/AKI+ 0.90 (0.81-0.99) < .04 Monitoring–/AKI– 0.63 (0.56-0.70) < .001 Monitoring+/AKI– 0.57 (0.48-0.68) < .001 Age by 5 y 1.12 (1.11-1.14) < .001 APS-III score by 10 units 1.20 (1.18-1.21) < .001
- 36. 尿量モニタリングと死亡リスク 36 Kellum JA, et al. J Am Soc Nephrol. 2015 Sep;26(9):2231-8. PMID: 25568178 Retrospective cohort, Multiple ICU (mixed), one large academic medical center, 2000-2008, 32,045 patients, AKI diagnosis; KDIGO criteria UO, SCr単独よりもUO+SCrの?がより?存予後予測できる SC: serum creatinine No AKI Stage 1 Stage 2 Stage 3 P Value Surgical Admission 6,202 (60.3) 2,903 (59.4) 6,800 (63.2) 2,441 (59.5) <0.001 Vasopressors, 034(18.4) 1,276(24.5) 3,040(26.5) 1,929(44.7) <0.001 Mechanical Ventilation 403(0.5) 3,068(58.8) 7,551(65.9) 3,043(70.6) <0.001 Suspected sepsis 774(7) 564(10.8) 1,540(13.4) 978(22.7) <0.001 KDIGO Stage UO Only No AKI Stage 1 Stage 2 Stage 3 Total No AKI 8,179 3,158 5,421 440 17,198 Dead 4.30% 5.30% 7.90% 17.70% 5.90% RRT 0.00% 0.00% 0.10% 1.10% 0.10% Stage 1 1,889 1,262 3,485 842 7,478 Dead 8.00% 11.30% 13.00% 32.10% 13.60% RRT 0.30% 0.70% 0.60% 10.90% 1.70% SC Only Stage 2 618 476 1,533 831 3,458 Dead 11.30% 23.90% 21.50% 44.20% 25.50% RRT 1.00% 1.30% 1.70% 21.70% 6.30% Stage 3 371 321 1,019 2,200 3,911 Dead 11.60% 38.60% 28.00% 51.10% 40.30% RRT 3.20% 17.80% 14.20% 55.30% 36.60% Total 11,057 5,217 11,458 4,313 32,045 Dead 5.60% 10.50% 13.00% 42.60% 14.00% RRT 0.30% 1.40% 1.70% 34.60% 5.60% Group 1 Group 2 Group 3 Group 4 Group 5 Group 6
- 37. 尿量モニタリングと死亡リスク 37 Bianchi NA, et al. JAMA Netw Open. 2021 Nov 1;4(11):e2133094. PMID: 34735011.. Retrospective cohort, single center, tertiary ICU, 15,620 patients, ≥18 years, admitted for more than 6 hours,, January 1, 2010, to June 15, 2020 SCrとUOの?致率は低い weighted κ coefficient, 0.36 [0.35-0.37; P < .001] 全体の比率 UO 低下à死亡リスク増加 Characteristic All No AKI AKI, sCr plus UO AKI, UO only AKI, sCr only (N = 15 620) (n = 3477) (n = 5524) (n = 5630) (n = 989) Septic shock 1762 (11.6) 181 (5.3) 1044 (19.5) 297 (5.4) 240 (25.0) Neurological 1615 (10.6) 456 (13.4) 380 (7.1) 667 (12.1) 112 (11.7) Surgical 8724 (57.2) 1923 (56.6) 2888 (53.7) 3488 (63.3) 425 (44.0) Medical 6536 (42.8) 1476 (43.4) 2492 (46.3) 2026 (36.7) 542 (56.0) Elective admission 4524 (29.6) 1187 (34.9) 978 (18.2) 2242 (40.7) 117 (12.1)
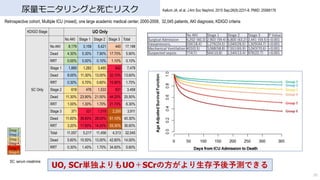
- 38. AKIの診断 尿量 + SCr ↓ 予後予測の精度が高い 38 慈恵医?ICU勉強会 2017年6?6?AKIの診断における尿量の意義 参照
- 39. 周術期の尿量と術後AKI (??臓?術) 39 Myles PS, et al. Br J Anaesth. 2019 Feb 15. [Epub ahead of print] PMID: 30916001 RCT, RELIEF trialのpost hoc, 術中乏尿と術後AKIとの関連を検証 腹部?術症例 (?腸約50%, ?道?泌尿器?婦?科各々約10%, 緊急?術約8%) Oliguria (N=889) vs. No_oliguria (N=1528), oliguria: urine output <0.5 mL/kg/h for at least 1hr 術後AKIリスク 術中乏尿 OR: 1.29 [1.14 ? 1.45, p<0.001] Factor AKI RR (95% CI) P-value Treatment group interaction All patients (n=2354) Any oliguria Yes (n=864) 220 (26) 1.29 (1.14-1.45) <0.001 <0.001 No (n=1490) 274 (18) reference Urine output (mL/kg/h) 0.006* <0.001 ≥0.5 (n=1880) ) 366 (20) reference - - 0.40-0.49 (n=143) 39 (27) 1.50 (1.05-2.13) 0.025 0.5 0.30-0.39 (n=128) 36 (28) 1.56 (1.08-2.26) 0.018 0.14 0.20-0.29 (n=102) 33 (32) 1.90 (1.27-2.82) 0.002 0.077 0.10-0.19 (n=71) 14 (20) 1.02 (0.57-1.80) 0.96 0.84 <0.10 (n=31) 7 (23) 1.20 (0.52-2.77) 0.67 0.81 AUC 0.54 [0.51 ? 0.56] Positive predictive value: 25.5% Negative predictive value: 81.6%
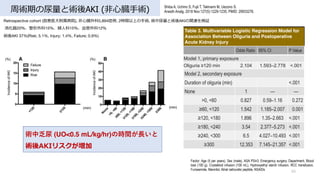
- 40. 周術期の尿量と術後AKI (??臓?術) 40 Shiba A, Uchino S, Fujii T, Takinami M, Uezono S. Anesth Analg. 2018 Nov;127(5):1229-1235. PMID: 29933276. Retrospective cohort (慈恵医?附属病院), ??臓外科5,894症例, 2時間以上の?術, 術中尿量と術後AKIの関連を検証 消化器20%、整形外科15%、婦?科15%、?管外科12% 術後AKI 37%(Risk; 5.1%, Injury; 1.4%, Failure; 0.9%) 術中乏尿 (UO<0.5 mL/kg/hr)の時間が?いと 術後AKIリスクが増加 Table 3. Multivariable Logistic Regression Model for Association Between Oliguria and Postoperative Acute Kidney Injury Factor: Age (5 per years), Sex (male), ASA PS≥3, Emergency surgery, Department, Blood loss (100 g), Crystalloid infusion (100 mL), Hydroxyethyl starch infusion, RCC transfusion, Furosemide, Mannitol, Atrial natriuretic peptide, NSAIDs Odds Ratio 95% CI P Value Model 1, primary exposure Oliguria ≥120 min 2.104 1.593–2.778 <.001 Model 2, secondary exposure Duration of oliguria (min) <.001 None 1 — — >0, <60 0.827 0.59–1.16 0.272 ≥60, <120 1.542 1.185–2.007 0.001 ≥120, <180 1.896 1.35–2.663 <.001 ≥180, <240 3.54 2.377–5.273 <.001 ≥240, <300 6.5 4.027–10.493 <.001 ≥300 12.353 7.145–21.357 <.001
- 41. 周術期の尿量と術後AKI (?臓?術) 41 Song Y, et al. Medicine (Baltimore). 2016 May;95(22):e3757. PMID: 27258505 . Retrospective cohort, ?臓外科696症例 (術後AKI 37%), CPB中の尿量と術後AKIの関連を検証 CPB中UO< 4 mL/kg/hr 術後AKIリスクが増加 CPB UO< 4 mL/kg/hr CPB UO ≥4 mL/kg/hr UO 4 mL/kg/hr CPB Urine Output mL/kg/hr Variables No AKI (n=439) AKI (n=257) P Type of surgery Mitral valve surgery only 201 (46) 136 (53) 0.69 Aortic valve surgery only 141 (32) 89 (35) 0.497 Double valve operation 42 (10) 52 (20) <.001 Coronary valve operation 31 (7) 27 (11) 0.113 Aorta surgery 17 (4) 15 (6) 0.448 Adult congenital 21 (5) 15 (6) 0.545 Transplantation 1 (0) 3 (1) 0.145 Others 28 (6) 24 (9) 0.152 CPB time, min 105 [80–135] 127 [91–161] <0.001
- 42. 周術期の尿量と術後AKI (?臓?術) 42 Pet?j? L, et al. J Cardiothorac Vasc Anesth. 2017 Jun;31(3):827-836. PMID: 27856153 . Single-center, prospective cohort, ?臓外科術後638症例, 術後AKIと予後を検証 AKI: 28.7% (KDIGO: 1; 18.8%, 2;6.1%, 3; 3.8%), No AKI: 71.3% 術後AKI UO+SCrで診断された?が死亡リスク?い HR: 4.9 [2.4-9.9] 術後AKIリスクファクター Multivariable Odds Ratio (95% CI) p Value Age,years 1.02(1.00-1.04) 0.027 Body mass index 430 kg/m2, yes/no 2.99 (1.87-4.78) 0.001 Chronic kidney disease, yes/no 4.08 (2.22-7.50) 0.001 Peripheralvascular disease, yes/no 1.95 (1.10-3.45) 0.023 Urgent/emergency versus elective surgery 1.76 (1.11-2.78) 0.017 Lowest hematocrit, % 0.95(0.92-0.99) 0.009 Vasopressor load, ?g/kg/min 3.72 (1.50-9.25) 0.005 Inodilator therapy, yes/no 2.49 (1.54-4.02) 0.001 Furosemide in intensive care unit, yes/no 3.84(2.47-5.99) 0.001 Multivariable Regression Analysis of Covariates Predicting AKI During First 5 Postoperative Days
- 45. AKIの診断 循環評価 45
- 46. 尿量をターゲットにした循環管理 46 van der Zee EN, et al. BMC Anesthesiol. 2017 Feb 10;17(1):22. Systematic Review. PMID: 28187752 尿量をターゲットにした循環管理が死亡割合に関連するかを検証, Systematic review, 36 RCT CFM: Conventional Fluid Therapy Mortality risk OR [95% CI] I2 Not Targeting UO in both protocol (13 RCT) 0.87 [0.59 - 1.27, p=0.94] 20% Targeting UO only in CFM (7 RCT) 1.09 [0.59 – 2.00, p=0.91] 4.7% Targeting UO in both protocol (16 RCT) 0.77 [0.59 - 1.01, p=0.47] 35.6% Total (36 RCT) 0.825 [0.684 - 0.995, p=0.95] 28% 7RCTでGDT群で尿量を指標にしていなかったが、 死亡リスクは変わらなかった 尿量測定の30?死亡リスク 死亡との関連は?せず 尿量を輸液管理の指標として死亡割合との関連を直接検証した 研究はなかった GDT vs. CFMの研究 尿量測定と30?死亡割合を提?している研究を選択
- 48. 尿排出 48
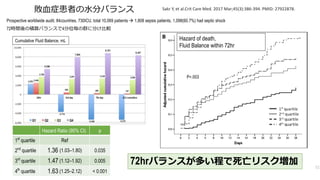
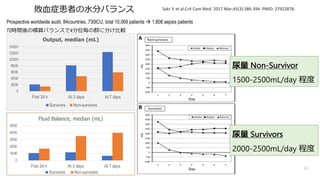
- 51. Prospective worldwide audit, 84countries, 730ICU, total 10,069 patients à 1,808 sepsis patients, 1,098(60.7%) had septic shock 72時間後の積算バランスで4分位毎の群に分け?較 Sakr Y, et al.Crit Care Med. 2017 Mar;45(3):386-394. PMID: 27922878. Hazard of death, Fluid Balance within 72hr P=.003 Cumulative Fluid Balance, mL 72hrバランスが多い程で死亡リスク増加 51 敗?症患者の?分バランス Hazard Ratio (95% CI) p 1st quartile Ref 2nd quartile 1.36 (1.03–1.80) 0.035 3rd quartile 1.47 (1.12–1.92) 0.005 4th quartile 1.63 (1.25–2.12) < 0.001
- 52. Prospective worldwide audit, 84countries, 730ICU, total 10,069 patients à 1,808 sepsis patients 72時間後の積算バランスで4分位毎の群に分け?較 Sakr Y, et al.Crit Care Med. 2017 Mar;45(3):386-394. PMID: 27922878. 52 敗?症患者の?分バランス 0 2000 4000 6000 8000 10000 12000 14000 First 24 h At 3 days At 7 days Output, median (mL) Survivors Non-survivors 0 1000 2000 3000 4000 5000 First 24 h At 3 days At 7 days Fluid Balance, median (mL) Survivors Non-survivors 尿量 Non-Survivor 1500-2500mL/day 程度 尿量 Survivors 2000-2500mL/day 程度
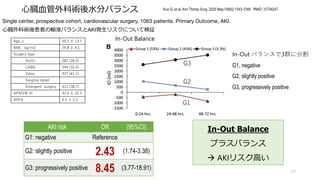
- 53. ?臓?管外科術後?分バランス 53 Kuo G, et al. Ann Thorac Surg. 2020 May;109(5):1343-1349. PMID: 31734247. Single center, prospective cohort, cardiovascular surgery, 1063 patients. Primary Outcome, AKI. ?臓外科術後患者の輸液バランスとAKI発?リスクについて検証 Age, y 59.1 ? 13.7 BMI, kg/m2 24.8 ? 4.1 Surgery type Aortic 282 (26.5) CABG 344 (32.4) Valve 437 (41.1) Surgical detail Emergent surgery 411 (38.7) APACHE III 43.5 ? 22.3 SOFA 6.5 ? 2.2 In-Out バランスで3群に分割 G1, negative G2, slightly positive G3, progressively positive AKI risk OR (95%CI) G1: negative Reference G2: slightly positive 2.43 (1.74-3.38) G3: progressively positive 8.45 (3.77-18.91) In-Out Balance プラスバランス à AKIリスク?い In-Out Balance G1 G2 G3
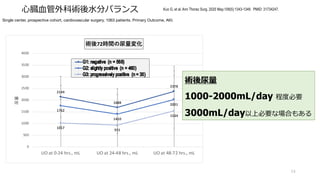
- 54. ?臓?管外科術後?分バランス 54 Kuo G, et al. Ann Thorac Surg. 2020 May;109(5):1343-1349. PMID: 31734247. Single center, prospective cohort, cardiovascular surgery, 1063 patients. Primary Outcome, AKI. 2144 1688 2378 1762 1410 2031 1017 931 1534 0 500 1000 1500 2000 2500 3000 3500 4000 UO at 0-24 hrs., mL UO at 24-48 hrs., mL UO at 48-72 hrs., mL 尿量 術後72時間の尿量変化 術後尿量 1000-2000mL/day 程度必要 3000mL/day以上必要な場合もある
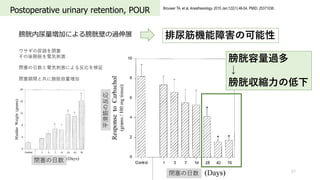
- 57. Postoperative urinary retention, POUR 57 Brouwer TA, et al. Anesthesiology. 2015 Jan;122(1):46-54. PMID: 25371036. 膀胱内尿量増加による膀胱壁の過伸展 ウサギの尿路を閉塞 その後膀胱を電気刺激 閉塞の?数と電気刺激による反応を検証 閉塞期間と共に膀胱容量増加 排尿筋機能障害の可能性 膀胱容量過多 ↓ 膀胱収縮?の低下 閉塞の?数 閉塞の?数 平滑筋の反応
- 58. 膀胱内容量の限界は? 58
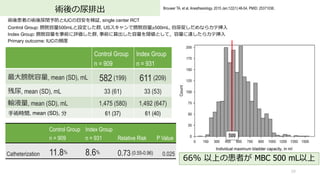
- 59. 術後の尿排出 59 Brouwer TA, et al. Anesthesiology. 2015 Jan;122(1):46-54. PMID: 25371036. 術後患者の術後尿閉予防とIUCの?安を検証, single center RCT Control Group: 膀胱容量500mLと設定した群, USスキャンで膀胱容量≥500mL, ?尿促しだめならカテ挿? Index Group: 膀胱容量を事前に評価した群, 事前に算出した容量を閾値として,容量に達したらカテ挿? Primary outcome: IUCの頻度 Control Group n = 909 Index Group n = 931 Relative Risk P Value Catheterization 11.8% 8.6% 0.73 (0.55-0.96) 0.025 66% 以上の患者が MBC 500 mL以上 500 Control Group n = 909 Index Group n = 931 最?膀胱容量, mean (SD), mL 582 (199) 611 (209) 残尿, mean (SD), mL 33 (61) 33 (53) 輸液量, mean (SD), mL 1,475 (580) 1,492 (647) ?術時間, mean (SD), 分 61 (37) 61 (40)
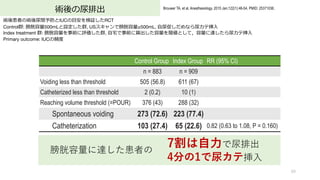
- 60. 術後の尿排出 60 Brouwer TA, et al. Anesthesiology. 2015 Jan;122(1):46-54. PMID: 25371036. 術後患者の術後尿閉予防とIUCの?安を検証したRCT Control群: 膀胱容量500mLと設定した群, USスキャンで膀胱容量≥500mL, ?尿促しだめなら尿カテ挿? Index treatment 群: 膀胱容量を事前に評価した群, ?宅で事前に算出した容量を閾値として,容量に達したら尿カテ挿? Primary outcome: IUCの頻度 Control Group Index Group RR (95% CI) n = 883 n = 909 Voiding less than threshold 505 (56.8) 611 (67) Catheterized less than threshold 2 (0.2) 10 (1) Reaching volume threshold (=POUR) 376 (43) 288 (32) Spontaneous voiding 273 (72.6) 223 (77.4) Catheterization 103 (27.4) 65 (22.6) 0.82 (0.63 to 1.08, P = 0.160) 7割は??で尿排出 4分の1で尿カテ挿? 膀胱容量に達した患者の
- 62. 硬膜外カテーテルの影響は? 62
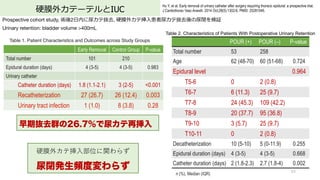
- 63. 硬膜外カテーテルとIUC 63 Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med. 2009 Nov-Dec;34(6):542-8. PMID: 19916208. Single center RCT, 硬膜外カテーテル使?した腹部外科患者215例 IUC早期抜去群, ?術翌朝に尿カテ抜去,標準治療群, 硬膜外カテ挿?抜去まで尿カテ留置 硬膜外カテーテル挿?患者の尿カテ早期抜去 術後UTI少ない 尿カテ再挿?に有意差なし ICU早期抜去 N=105 標準治療群 N=110 p-value Age, y (SD) 57 (15) 63 (11) 0.002 Age ≥65 y. % (n) 34 (36) 41(45) 0.32 Men, % 52 62 0.1 24 Type of Surgery Thoracic, % 47 51 0.53 Abdominal, % 53 50 Duration of surgery, min (SD) 236 (95) 271 (111) 0.02 ICU早期抜去 N=105 標準治療群 N=110 P-value UTI, N 2 15 0.004 尿カテ再留置, N 8 2 0.09 間欠的導尿, N 3 0 0.229
- 64. Early Removal Control Group P-value Total number 101 210 Epidural duration (days) 4 (3-5) 4 (3-5) 0.983 Urinary catheter Catheter duration (days) 1.8 (1.1-2.1) 3 (2-5) <0.001 Recatheterization 27 (26.7) 26 (12.4) 0.003 Urinary tract infection 1 (1.0) 8 (3.8) 0.28 硬膜外カテーテルとIUC 64 Hu Y, et al. Early removal of urinary catheter after surgery requiring thoracic epidural: a prospective trial. J Cardiothorac Vasc Anesth. 2014 Oct;28(5):1302-6. PMID: 25281046. Prospective cohort study, 術後2?内に尿カテ抜去, 硬膜外カテ挿?患者尿カテ抜去後の尿閉を検証 Urinary retention: bladder volume >400mL 早期抜去群の26.7%で尿カテ再挿入 Table 1. Patient Characteristics and Outcomes across Study Groups n (%), Median (IQR) POUR (+) POUR (–) P-value Total number 53 258 Age 62 (48-70) 60 (51-68) 0.724 Epidural level 0.964 T5-6 0 2 (0.8) T6-7 6 (11.3) 25 (9.7) T7-8 24 (45.3) 109 (42.2) T8-9 20 (37.7) 95 (36.8) T9-10 3 (5.7) 25 (9.7) T10-11 0 2 (0.8) Decatheterization 10 (5-10) 5 (0-11.9) 0.255 Epidural duration (days) 4 (3-5) 4 (3-5) 0.668 Catheter duration (days) 2 (1.8-2.3) 2.7 (1.8-4) 0.002 Table 2. Characteristics of Patients With Postoperative Urinary Retention 硬膜外カテ挿?部位に関わらず 尿閉発生頻度変わらず
- 65. 硬膜外カテーテルとIUC 65 Young J, et al. J Thorac Cardiovasc Surg. 2018 Jul;156(1):430-435. PMID: 29609886. Retrospective cohort, 胸部硬膜外カテーテル挿?した胸部?術患者275症例 SCIP9に則ってUC抜去?多変量解析で再挿?リスクを検証 48時間以内のUC再挿?; あり (21.6%) vs. なし(78.4%) 尿カテ再挿?リスク因?:?道?術, BPH Factors P value OR (95% CI) Esophageal resection (vs pulmonary) <.001 4.16 (2.05-8.43) Presence of BPH* Female 0.056 0.49 (0.24-1.02) Male without BPH reference Male with BPH 0.049 2.73 (1.00-7.45) Lower BMI 0.029 0.93 (0.87-0.99) Age 0.07 1.03 (0.99-1.06)
- 66. 硬膜外脊髄くも膜下併??酔とIUC 66 Thiengwittayaporn S, et al. Arch Orthop Trauma Surg. 2021 Mar;141(3):469-476. PMID: 33180187. Design: single center RCT. Patient: TKA, CSEA. 硬膜外カテーテルは24時間以内に抜去 retained UC群 (UC, N=113), non-retained UC群 (non-UC, N=113) groups Outcome: incidences of postoperative urinary retention (POUR, <400mL) Clinical outcomes UC (N = 113) Non-UC (N = 113) p value POUR, n 3 6 0.4 Mean intraoperative intravenous fluid, ml (range) 1054.0 (350–1300) 978.8 (300–1500) 0.07 Mean duration of the surgery, min (range) 89.5 (54–149) 89.8 (60–140) 0.8 UTI, n 2 0 0.4 Mean length of stay, day (range) 4.8 (3–10) 4.5 (2–9) 0.08 硬膜外脊髄くも膜下併??酔患者 術中輸液1000mL程度 à 術後に尿カテがなくても 術後尿閉有意差なし ?術時間90分程度
- 68. 合併症 68
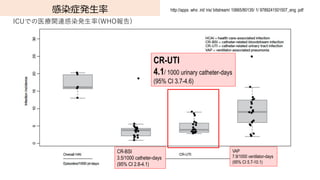
- 71. 感染症発?率 CR-UTI 4.1/ 1000 urinary catheter-days (95% CI 3.7-4.6) ICUでの医療関連感染発?率(WHO報告) http://apps .who .int/ iris/ bitstream/ 10665/80135/ 1/ 9789241501507_eng .pdf VAP 7.9/1000 ventilator-days (95% CI 5.7-10.1) CR-BSI 3.5/1000 catheter-days (95% CI 2.8-4.1)
- 73. IUC合併症 73 Saint S, et al. JAMA Intern Med. 2018 Aug 1;178(8):1078-1085. PMID: 29971436; PMCID: PMC6143107. Infectious Complication Non-Infectious Complication Variable IRR (95% CI) P Value IRR (95% CI) P Value Urinary Catheter Duration 3 days or Less 1 [Reference] 1 [Reference] More than 3 days 1.38 (1.03-1.84) 0.03 1.27 (1.17-1.37) <.001 Other Factor, Sex; Reason; AUA Symptom Index Score; Age Percentage of 2076 Patients Reporting Complications After IUC Multivariable Linear Regression Model to Predict Infectious and Noninfectious Urethral Catheter Complications 3?以上の尿カテ挿?は 合併症のリスク ?感染性合併症 感染症の5倍以上 Total [55.4%] Woman [47.3%] Men [58.6%] P < .001 Total [10.5%] Women [15.5%] Men [ 8.6%] P < .001
- 74. ?感染性IUC合併症 74 Hollingsworth JM, et al. Meta-analysis,37studies, 2868 patients 合併症 頻度 95% CI 尿漏れ 10.6% [2.4 to 17.7] 尿道狭窄(Higher quality studies) 16.7% [13.0 to 20.8] (Low quality studies) 3.4% [1.0 to 7.0] 血尿 4.7% [0.0 to 10.0] 事故抜去 4.0% [0.0 to 8.6] 留置中 !"#$%&' ( 感染性合併症 15.3% ?感染性合併症 70.2% 痛み?は不快感 54.5% 切迫感や膀胱の痙攣 34.7% ?尿 27.4% ?膚障害 19.4% その他の合併症 社会活動の制限 43.9% ?常?活の制限 39.5% 抜去後 (N=2034) % 尿漏れ 20.3% 排尿開始または尿?め困難 19.5% 排尿時痛または灼熱感 17.4% 排尿困難 12.1% 尿の噴き出し 9.2% ?膚障害 6.6% 挿?部の出? 4.6% Saint S, et al. Prospective cohort, 4 US Hospital, 2076 patients with IUC.内科/外科急性期病棟, ICU 18歳以上、参加者に聴取し3?以内に尿カテ留置した患者、留置から14??と30??詳細聴取 ?感染性合併症 約7割 Hollingsworth JM, et al. Ann Intern Med. 2013 Sep 17;159(6):401-10. PMID: 24042368. Saint S, et al. JAMA Intern Med. 2018 Aug 1;178(8):1078-1085. PMID: 29971436; PMCID: PMC6143107.
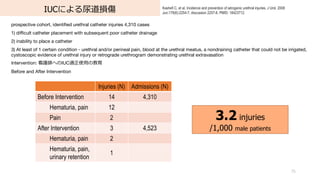
- 75. IUCによる尿道損傷 75 Kashefi C, et al. Incidence and prevention of iatrogenic urethral injuries. J Urol. 2008 Jun;179(6):2254-7; discussion 2257-8. PMID: 18423712. prospective cohort, identified urethral catheter injuries 4,310 cases 1) difficult catheter placement with subsequent poor catheter drainage 2) inability to place a catheter 3) At least of 1 certain condition - urethral and/or perineal pain, blood at the urethral meatus, a nondraining catheter that could not be irrigated, cystoscopic evidence of urethral injury or retrograde urethrogram demonstrating urethral extravasation Intervention: 看護師へのIUC適正使?の教育 Before and After Intervention Injuries (N) Admissions (N) Before Intervention 14 4,310 Hematuria, pain 12 Pain 2 After Intervention 3 4,523 Hematuria, pain 2 Hematuria, pain, urinary retention 1 3.2 injuries /1,000 male patients
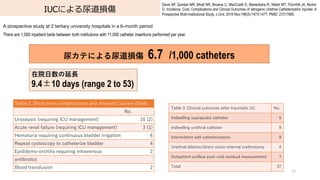
- 76. IUCによる尿道損傷 76 Davis NF, Quinlan MR, Bhatt NR, Browne C, MacCraith E, Manecksha R, Walsh MT, Thornhill JA, Mulvin D. Incidence, Cost, Complications and Clinical Outcomes of Iatrogenic Urethral Catheterization Injuries: A Prospective Multi-Institutional Study. J Urol. 2016 Nov;196(5):1473-1477. PMID: 27317985. A prospective study at 2 tertiary university hospitals in a 6-month period There are 1,000 inpatient beds between both institutions with 11,000 catheter insertions performed per year. 尿カテによる尿道損傷 6.7 /1,000 catheters 在院?数の延? 9.4±10 days (range 2 to 53) Table 3. Clinical outcomes after traumatic UC No. Indwelling suprapubic catheter 9 Indwelling urethral catheter 8 Intermittent self-catheterization 9 Urethral dilation/direct vision internal urethrotomy 4 Outpatient uroflow post-void residual measurement 7 Total 37 Table 2. Short-term complications and relevant Clavien-Dindo No. Urosepsis (requiring ICU management) 10 (2) Acute renal failure (requiring ICU management) 3 (1) Hematuria requiring continuous bladder irrigation 6 Repeat cystoscopy to catheterize bladder 4 Epididymo-orchitis requiring intravenous 2 antibiotics Blood transfusion 2
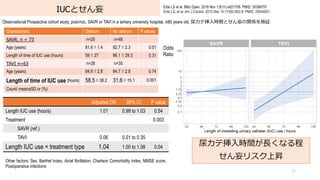
- 77. IUCとせん妄 77 Eide LS et al. BMJ Open. 2018 Nov 1;8(11):e021708. PMID: 30389757 Eide LS, et al. Am J Cardiol. 2015 Mar 15;115(6):802-9. PMID: 25644851. Observational Prospective cohort study, post-hoc, SAVR or TAVI in a tertiary university hospital, ≥80 years old, 尿カテ挿?時間とせん妄の関係を検証 尿カテ挿?時間が?くなる程 せん妄リスク上昇 Other factors: Sex, Barthel Index, Atrial fibrillation, Charlson Comorbidity Index, MMSE score, Postoperative infections Adjusted OR 95% CI P value Length IUC use (hours) 1.01 0.99 to 1.03 0.54 Treatment 0.003 SAVR (ref.) TAVI 0.06 0.01 to 0.35 Length IUC use × treatment type 1.04 1.00 to 1.08 0.04 Characteristic Delirium No delirium P values SAVR, n = 73 n=25 n=48 Age (years) 81.6±1.4 82.7±2.3 0.01 Length of time of IUC use (hours) 59±27 66.1±29.3 0.31 TAVI n=63 n=28 n=35 Age (years) 84.9±2.8 84.7±2.8 0.74 Length of time of IUC use (hours) 58.5±38.2 31.6±15.1 0.001 Count/ mean±SD or (%) Odds Ratio
- 78. Retrospective cohort, 慈恵医?葛飾医療センター, 低侵襲?術患者5112例, 術後の意識変容を解析。診察記録からせん妄と思われる状態を抽出。 Inverse Probability Weightingで調整 意識変容リスク Fukushima T, et al. Ann Med Surg (Lond). 2021 Mar 6;64:102186. PMID: 33747493 IUCとせん妄 All Patients Inverse Propensity-Weighted Patients Control IUC ASD Control IUC ASD Number of patients, N (%) 2,249 2,863 3,080.94 2,863.00 Age, y 48 [36 - 63] 60 [44 - 72] 0.477 60 [47 - 71] 60 [44 - 72] 0.024 Surgery type, N (%) 0.498 0.089 General surgery* , N (%) 633 (28.1) 1,283 (44.8) 1,393.3 (45.2) 1,283.0 (44.8) ENT surgery? , N (%) 820 (36.5) 498 (17.4) 457.3 (14.8) 498.0 (17.4) Orthopedic surgery? , N (%) 724 (32.2) 896 (31.3) 979.3 (31.8) 896.0 (31.3) Others, N (%) 72 (3.2) 186 (6.5) 251.1 (8.1) 186.0 (6.5) Age >65 y, Male,BMI >30 kg/m2,Surgery type, Nerve block, Fentanyl use Odds ratio [95% CI] All patients P-value IPW P-value Urinary catheter 2.08[1.44 - 3.03] <0.001 1.97 [1.50 - 2.59] <0.001 Inverse Propensity-Weighted Patients Control IUC ASD P-value N 3,080.94 2,863.00 AMS or UTI 98.3 (3.2) 143.0 (5.0) 0.091 0.397 AMS, Altered Mental Status 尿カテ挿? ↓ 意識変容のリスク因?
- 79. IUCによる尿道狭窄 79 Prospective cohort, 尿道狭窄形成術を?った268症例の解析 治療対象の11.2 % がIUCによる影響 Lumen N, et al. J Urol. 2009 Sep;182(3):983-7. PMID: 19616805. Retrospective cohort, DVIUを?った医原性尿道狭窄224症例の解析 (DVIU: direct vision internal urethrotomy, 経尿道的内尿道切開術) IUCによる尿道狭窄は31.7%, そのうち再発は26.5% K?z?lay F, et al. Turk J Med Sci. 2017 Nov 13;47(5):1543-1548. PMID: 29151330.
- 80. 尿道狭窄ガイドライン 80 Lumen N et al. Eur Urol. 2021 Aug;80(2):190-200. PMID: 34059397. Campos-Juanatey F, et al. Eur Urol. 2021 Aug;80(2):201-212. PMID: 34103180. European Association of Urology Guidelines on Urethral Stricture Disease Recommendations Strength rating Advise safe sexual practices, recognise symptoms of sexually transmitted infections, and provide access to prompt investigation and treatment for men with urethritis. Strong Avoid unnecessary urethral catheterisation. Strong Implement training programmes for physicians and nurses performing urinary catheterisation. Strong Do not use catheters larger than 18 Fr if urinary drainage only is the purpose Weak Avoid using noncoated latex catheters Strong Do not perform urethrotomy routinely when there is no pre-existent urethral stricture Strong
- 82. Indwelling vs. Intermitted 82 Kidd EA, et al. Cochrane Database Syst Rev. 2015 Dec 10;(12):CD004203. PMID: 26661940.
- 83. Indwelling vs. Intermitted 83 Kidd EA, et al. Cochrane Database Syst Rev. 2015 Dec 10;(12):CD004203. PMID: 26661940.
- 85. 代替?法 85
- 86. 超?波診断装置 体重測定 86
- 87. 超?波診断装置 体重測定 87
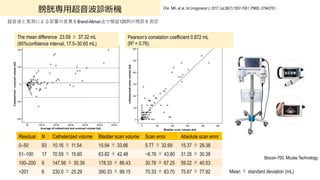
- 88. 膀胱専?超?波診断装置による測定 88 周術期IUCの必要な患者、超?波内容量測定した後、尿カテ挿?し尿量測定 Brouwer TA, et al. J Clin Monit Comput. 2018 Dec;32(6):1117-1126. PMID: 29516310 Verathon Medical BVI 9400? BVI 9400 (n = 105) Estimated volume, mean (SD) (mL) 288 (237.0) Actual volume, mean (SD) (mL) 266 (241.9) Difference estimated–actual volume, mean (SD) (mL) 21.8 (59.9) Preoperative measurement, no (%) 89 (84.8) Actual volumes > 400 mL, no (%) 26 (24.8) Actual volumes > 500 mL, no (%) 17 (16.2) Actual volumes > 600 mL, no (%) 11 (10.5) Volumes ≤400mL Volumes >400 mL N Bias LOA N Bias LOA BVI 9400 79 25.7 ? 69 to 121 26 10 ? 159 to 179 400 mL 500 mL 600 mL True negatives True positives True negatives True positives True negatives True positives BVI 9400 100 (1.00–1.00) 89.7 (0.79–1.00) 100 (1.00–1.00) 90.9 (0.79–1.00) 98.9 (0.97–1.00) 80.0 (0.60–1.00) LOA; Limit of Agreement (±1.96SD)
- 89. 膀胱専?超?波診断機 89 Brouwer TA, et al. J Clin Monit Comput. 2018 Dec;32(6):1117-1126. PMID: 29516310 Daurat A, et al. Anesth Analg. 2015 May;120(5):1033-1038. PMID: 25642660. Cho MK, et al. Int Urogynecol J. 2017 Jul;28(7):1057-1061. PMID: 27942791. Verathon Medical Bladderscan Prime plus? Verathon Medical Bladderscan BVI 3000? Biocon-700, Mcube Technology
- 90. 膀胱専?超?波診断機 Cho MK, et al. Int Urogynecol J. 2017 Jul;28(7):1057-1061. PMID: 27942791. 超?波と実測による尿量の差異をBrand-Altman法で検証125例の残尿を測定 Biocon-700, Mcube Technology Residual N Catheterized volume Bladder scan volume Scan error Absolute scan error 0–50 93 10.16 ± 11.54 15.94 ± 33.66 5.77 ± 32.69 15.37 ± 29.38 51–100 17 70.59 ± 15.60 63.82 ± 42.48 ?6.76 ± 43.80 31.35 ± 30.38 100–200 9 147.56 ± 30.39 178.33 ± 86.43 30.78 ± 67.25 59.22 ± 40.53 >201 6 230.0 ± 25.29 300.33 ± 99.15 70.33 ± 83.70 75.67 ± 77.92 Mean ± standard deviation (mL) Pearson’s correlation coefficient 0.872 mL (R2 = 0.76). The mean difference 23.59 ± 37.32 mL (95%confidence interval, 17.5–30.65 mL)
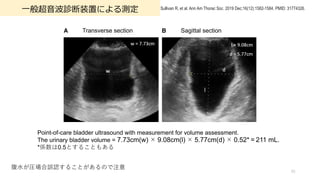
- 91. ?般超?波診断装置による測定 91 Sullivan R, et al. Ann Am Thorac Soc. 2019 Dec;16(12):1582-1584. PMID: 31774326. Point-of-care bladder ultrasound with measurement for volume assessment. The urinary bladder volume = 7.73cm(w)?×?9.08cm(l)?×?5.77cm(d)?×?0.52*?=?211 mL. *係数は0.5とすることもある 腹?が圧場合誤認することがあるので注意 Transverse section Sagittal section
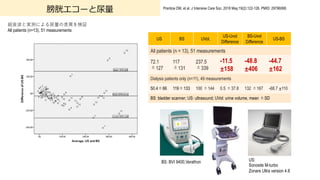
- 92. 膀胱エコーと尿量 Prentice DM, et al. J Intensive Care Soc. 2018 May;19(2):122-126. PMID: 29796068; 超?波と実測による尿量の差異を検証 All patients (n=13), 51 measurements US: Sonosite M-turbo Zonare Ultra version 4.8 BS: BVI 9400,Verathon US BS UVol, US-Uvol Difference BS-Uvol Difference US-BS All patients (n = 13), 51 measurements 72.1 ±127 117 ±131 237.5 ±339 -11.5 !158 -48.8 !406 -44.7 !162 Dialysis patients only (n=11), 49 measurements 50.4±66 119±133 100 ±144 0.5 ±37.8 132 ±167 -68.7 !110 BS: bladder scanner; US: ultrasound; UVol: urine volume, mean ±SD
- 93. 超?波診断装置 体重測定 93
- 94. 輸液バランスと体重 94 Testani JM, et al. Am J Med. 2015 Jul;128(7):776-83.e4 PMID: 25595470 急性?不全(ADHF)に対する利尿薬、尿量と体重変化の?致の程度を検証 A: DOSE (N Engl J Med. 2011;364:797-805.), B: ESCAPE (JAMA. 2005;294:1625-1633.), C: Penn (Circ Heart Fail. 2014;7:261-270.) データを?いたpost-hoc解析 (N=254) DOSE, Inpatient randomized trial of loop diuretic strategies in acute decompensated heart failure ESCAPE, Inpatient randomized trial of pulmonary artery catheters in acute decompensated heart failure Penn, Inpatient retrospective observational acute decompensated heart failure cohort A: DOSE B: ESCAPE C: Penn Correlation between net fluid and weight loss r=0.55, P < .001 r=0.48, P < .001 r=0.51, P < .001 95% limits of agreement (kg-L) -6.4 to 7.9 -7.5 to 11.6 -11.4 to 14.5 Mean bias (kg-L) 0.74±3.8 2.1±4.9 1.6±6.6 Mean of Change in Weight (Kg) and Net Fluid Loss (L) Change in Weight (Kg) - Net Fluid Loss (L) 尿量と体重変化 相関関係はあり 誤差は?きそう
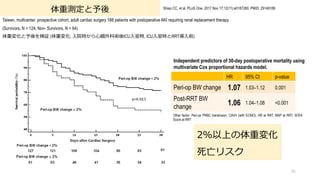
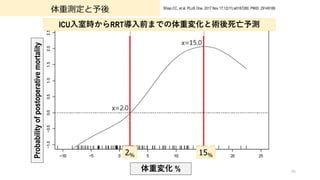
- 95. 体重測定と予後 Taiwan, multicenter, prospective cohort, adult cardiac surgery 188 patients with postoperative AKI requiring renal replacement therapy (Survivors, N = 124; Non- Survivors, N = 64) 体重変化と予後を検証 (体重変化: ?院時から?臓外科術後ICU?室時, ICU?室時とRRT導?前) 95 Shiao CC, et al. PLoS One. 2017 Nov 17;12(11):e0187280. PMID: 29149189 HR 95% CI p-value Peri-op BW change 1.07 1.03–1.12 0.001 Post-RRT BW change 1.06 1.04–1.08 <0.001 Independent predictors of 30-day postoperative mortality using multivariate Cox proportional hazards model. Other factor: Peri-op PRBC transfusion, CAVH (with ECMO), HR at RRT, MAP at RRT, SOFA Score at RRT 2%以上の体重変化 死亡リスク
- 96. 体重測定と予後 96 Shiao CC, et al. PLoS One. 2017 Nov 17;12(11):e0187280. PMID: 29149189 ICU?室時からRRT導?前までの体重変化と術後死亡予測 2% 15% 体重変化 % Probability of postoperative mortality
- 99. 改善?法 n ガイドライン/プロトコール作成 n スタッフの再教育 n チェックリスト n 包括院内システムの導入 n 行政制度?保険制度の導入
- 100. 100 Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619. 多施設コホート研究 32か国, 603施設, 926箇所 (non-ICUs; 59.7%, ICUs; 40.3%) UTI減少プログラムによるIUC適応の厳格化 UTI減少プロジェクト
- 101. UTI減少プロジェクト 101 Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619. プログラムによりUTI, IUC減少 UTI: 2.40 à 2.05 infections/1000 catheter-days, incidence rate ratio (IRR), 0.86 [0.76 to 0.96; P = 0.009] Non-ICU (N=553) Variable IRR (95% CI) P Value IRR (95% CI) P Value UTI Catheter Use Time 0.68 (0.56–0.82) <0.001 0.93 (0.90–0.96) <0.001 Teaching hospital 1.76 (1.03–3.01) 0.04 0.96 (0.73–1.26) 0.77 Rural hospital 0.90 (0.66–1.23) 0.51 0.89 (0.78–1.01) 0.07 CAH 2.36 (1.65–3.37) <0.001 0.95 (0.82–1.10) 0.47 Hospital size (per 100-bed increase) 0.97 (0.90–1.05) 0.45 0.98 (0.95–1.02) 0.38 UTI 2.28 à 1.54 infections/1000 catheter-days, IRR, 0.68 [0.56-0.82, P<0.001] IUC 20.1 à 18.8% IRR, 0.93 [0.90-0.96, P<0.001] CAH: Critical-access hospital 全体でUTI, 尿カテ使?率減少
- 102. UTI減少プロジェクト 102 Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619. UTI 2.48 à 2.50 infections/1000 catheter-days, IRR, 1.01 [0.87-1.17, P=0.90] IUC 62.8 à 61.9% IRR, 0.98 [0.96-1.01; P=0.15] プログラムによりUTI, IUC減少 UTI: 2.40 à 2.05 infections/1000 catheter-days, incidence rate ratio (IRR), 0.86 [0.76 to 0.96; P = 0.009] ICU (N=373) Variable IRR (95% CI) P Value IRR (95% CI) P Value UTI Catheter Use Time 1.01 (0.87–1.17) 0.9 0.98 (0.96–1.01) 0.15 Teaching hospital 1.92 (1.32–2.80) 0.001 0.96 (0.88–1.06) 0.45 Rural hospital 0.83 (0.58–1.18) 0.3 0.85 (0.78–0.91) <0.001 CAH 2.60 (0.94–7.20) 0.07 0.81 (0.67–0.98) 0.03 Hospital size (per 100-bed increase) 1.09 (1.02–1.16) 0.01 1.02 (1.01–1.04) 0.01 Rural Hospital, CAH à 尿カテ使?率 減少 CAH: Critical-access hospital
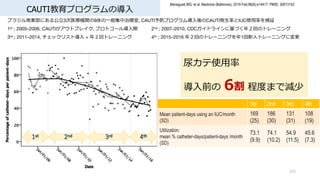
- 103. CAUTI教育プログラムの導? 103 Menegueti MG, et al. Medicine (Baltimore). 2019 Feb;98(8):e14417. PMID: 30813142 ブラジル南東部にある公?3次医療機関の9床の?般集中治療室, CAUTI予防プログラム導?後のCAUTI発?率とIUC使?率を検証 1st ; 2005–2006, CAUTIがアウトブレイク, プロトコール導?期 2nd ; 2007–2010, CDCガイドラインに基づく年2回のトレーニング 3rd ; 2011–2014, チェックリスト導? + 年2回トレーニング 4th ; 2015–2016 年2回のトレーニングを年1回新?トレーニングに変更 1st 2nd 3rd 4th Mean patient-days using an IUC/month (SD) 169 (25) 186 (30) 131 (31) 108 (19) Utilization: mean % catheter-days/patient-days /month (SD) 73.1 (9.9) 74.1 (10.2) 54.9 (11.5) 45.6 (7.3) 尿カテ使?率 導?前の 6割 程度まで減少
- 104. CAUTI教育プログラムの導? 104 Menegueti MG, et al. Medicine (Baltimore). 2019 Feb;98(8):e14417. PMID: 30813142 ブラジル南東部にある公?3次医療機関の9床の?般集中治療室, CAUTI予防プログラム導?後のCAUTI発?率とIUC使?率を検証 1st ; 2005–2006, CAUTIがアウトブレイク, プロトコール導?期 2nd ; 2007–2010, CDCガイドラインに基づく年2回のトレーニング 3rd ; 2011–2014, チェックリスト導? + 年2回トレーニング 4th ; 2015–2016 年2回のトレーニングを年1回新?トレーニングに変更 1st 2nd 3rd 4th CAUTI mean incidence rate, entire phase (No. of episodes/1000 catheter-days [SD]) 14.9 (7.5) 7.3 (5.0) 3. 8 (5.7) 1.1 (3.0) CAUTI 導?前の 7% 程度まで減少
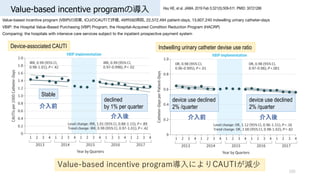
- 105. Value-based incentive programの導? 105 Hsu HE, et al. JAMA. 2019 Feb 5;321(5):509-511. PMID: 30721286 Value-based incentive program (VBIP)の効果, ICUのCAUTIで評価, 49州592病院, 22,572,494 patient-days, 13,607,240 indwelling urinary catheter-days VBIP: the Hospital Value-Based Purchasing (VBP) Program, the Hospital-Acquired Condition Reduction Program (HACRP) Comparing: the hospitals with intensive care services subject to the inpatient prospective payment system Value-based incentive program導?によりCAUTIが減少 device use declined 2% /quarter device use declined 2% /quarter 介?前 介?後 介?前 介?後 declined by 1% per quarter Stable Device-associated CAUTI Indwelling urinary catheter devise use ratio
- 106. 施設ガイドライン作成と救急室でのIUC 106 Fakih MG, et al. Acad Emerg Med. 2010 Mar;17(3):337-40. PMID: 20370769.. before-after, single center, 769 tertiatry care hospital, 2517 patients 施設ガイドライン作成による救急室でのIUC減少頻度を評価 Compliant with guidelines, n = 203 (63.0%) N (%) Non?intensive care 6 L /min oxygen 40 (12.4) Output monitoring in intensive care 39 (12.1) Emergent pelvic ultrasound 33 (10.2) Intubated 32 (9.9) Neurogenic bladder 14 (4.3) Emergency surgery 12 (3.7) Urinary obstruction 10 (3.1) Unresponsive 7 (2.2) Acute hip fracture 6 (1.9) Urologic procedures 4 (1.2) Acute mental status changes with agitation 4 (1.2) Stage 3 or 4 sacral decubitus ulcers with incontinence 1 (0.3) Hospice or palliative care 1 (0.3) Noncompliant with guidelines, n = 119 (37.0%) No clear reason 64 (19.9) Oxygen supplementation <6 L ? min 26 (8.1) Dementia 16 (5.0) Urine specimen collection 5 (1.6) Incontinence 3 (0.9) Patient request 3 (0.9) Output monitoring outside intensive care 2 (0.6) UC utilization 14.9% à 10.6% OR = 1.48, [95% CI 1.16-1.90, p = 0.002] ガイドライン非遵守IUCの理由 理由なしが約20%
- 107. 救急室でのIUC改善計画 107 Greene MT, et al. Infect Control Hosp Epidemiol. 2018 Jan;39(1):77-84. PMID: 29249212. Prospective observational study, 13,215 patients, 救急室での尿道カテーテル挿?改善計画 ?営利組織(Acscension)が経営する18施設; System-based, 16施設; ミシガン州によるState-based intervention I: CDCガイドラインを基にした尿カテ挿?基準を策定, C/O: 介?前後でのappropriate IUCの割合の変化 Intervention Phase UC Utilization, % P Valuea Appropriate UC, % P Valuea System-based hospitals Baseline 9.1 Ref 73.1 Ref Intervention 6.1 < .001 91 < .001 Sustainability 5.3 < .001 92.4 < .001 State-based hospitals Baseline 6.6 Ref 70.9 Ref Intervention 4.9 0.03 82 0.05 Sustainability 6.4 0.97 87.3 0.05 QI介?前 適正使?は7割程 QI介?後 適正使?は8-9割
- 109. 最後に 109
- 110. 110 Appropriate Urinary Catheter Use Need for accurate measurements of urinary output in critically ill patients. 尿道カテーテル留置のカイゼン活動の基準のほとんどはCDCガイドライン
- 111. 111 Appropriate Urinary Catheter Use Need for accurate measurements of urinary output in critically ill patients. 尿道カテーテル留置のカイゼン活動の基準のほとんどはCDCガイドライン タイミングは? 指標は? 対象症例は? 具体的な提示はない
- 112. SSCG 2021 Mean arterial pressure Recommendation 9. For adults with septic shock on vasopressors, we recommend an initial target mean arterial pressure (MAP) of 65 mm Hg over higher MAP targets Strong recommendation, moderate-quality evidence Monitoring and intravenous access Recommendations 43. For adults with septic shock, we suggest using invasive monitoring of arterial blood pressure over non-invasive monitoring, as soon as practical and if resources are available Weak recommendation, very low quality of evidence 44. For adults with septic shock, we suggest starting vasopressors peripherally to restore MAP rather than delaying initiation until a central venous access is secured Weak recommendation, very low quality of evidence 112
- 113. SSCG 2021 Mean arterial pressure Recommendation 9. For adults with septic shock on vasopressors, we recommend an initial target mean arterial pressure (MAP) of 65 mm Hg over higher MAP targets Strong recommendation, moderate-quality evidence Monitoring and intravenous access Recommendations 43. For adults with septic shock, we suggest using invasive monitoring of arterial blood pressure over non-invasive monitoring, as soon as practical and if resources are available Weak recommendation, very low quality of evidence 44. For adults with septic shock, we suggest starting vasopressors peripherally to restore MAP rather than delaying initiation until a central venous access is secured Weak recommendation, very low quality of evidence 113 尿量?尿道カテーテル 言及なし
- 114. ProCESS Protocolized Care for Early Septic Shock (United States, U.S.) ARISE Australasian Resuscitation In Sepsis Evaluation (Australia and New Zealand, ANZ) ProMISe Protocolised Management In Sepsis (United Kingdom, U.K) CVP, MAP, ScvO2を?いたEGDTを検証 114
- 115. ProCESS Protocolized Care for Early Septic Shock (United States, U.S.) ARISE Australasian Resuscitation In Sepsis Evaluation (Australia and New Zealand, ANZ) ProMISe Protocolised Management In Sepsis (United Kingdom, U.K) CVP, MAP, ScvO2を?いたEGDTを検証 115 尿量?尿道カテーテル 強固なエビデンスがない
- 117. Indwelling Urinary Catheter 117 適 応 に つ い て 真 剣 に 考 え る






















![尿量モニタリングと死亡リスク
37
Bianchi NA, et al. JAMA Netw Open. 2021 Nov 1;4(11):e2133094. PMID: 34735011..
Retrospective cohort, single center, tertiary ICU, 15,620 patients, ≥18 years, admitted for more than 6 hours,, January 1, 2010, to June 15, 2020
SCrとUOの?致率は低い
weighted κ coefficient, 0.36 [0.35-0.37; P < .001]
全体の比率
UO 低下à死亡リスク増加
Characteristic All No AKI AKI, sCr plus UO AKI, UO only AKI, sCr only
(N = 15 620) (n = 3477) (n = 5524) (n = 5630) (n = 989)
Septic shock 1762 (11.6) 181 (5.3) 1044 (19.5) 297 (5.4) 240 (25.0)
Neurological 1615 (10.6) 456 (13.4) 380 (7.1) 667 (12.1) 112 (11.7)
Surgical 8724 (57.2) 1923 (56.6) 2888 (53.7) 3488 (63.3) 425 (44.0)
Medical 6536 (42.8) 1476 (43.4) 2492 (46.3) 2026 (36.7) 542 (56.0)
Elective admission 4524 (29.6) 1187 (34.9) 978 (18.2) 2242 (40.7) 117 (12.1)](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-37-320.jpg)
![周術期の尿量と術後AKI (??臓?術)
39
Myles PS, et al. Br J Anaesth. 2019 Feb 15. [Epub ahead of print] PMID: 30916001
RCT, RELIEF trialのpost hoc, 術中乏尿と術後AKIとの関連を検証
腹部?術症例 (?腸約50%, ?道?泌尿器?婦?科各々約10%, 緊急?術約8%)
Oliguria (N=889) vs. No_oliguria (N=1528), oliguria: urine output <0.5 mL/kg/h for at least 1hr
術後AKIリスク
術中乏尿 OR: 1.29 [1.14 ? 1.45, p<0.001]
Factor AKI RR (95% CI) P-value
Treatment group
interaction
All patients (n=2354)
Any oliguria
Yes (n=864) 220 (26) 1.29 (1.14-1.45) <0.001 <0.001
No (n=1490) 274 (18) reference
Urine output (mL/kg/h) 0.006* <0.001
≥0.5 (n=1880) ) 366 (20) reference - -
0.40-0.49 (n=143) 39 (27) 1.50 (1.05-2.13) 0.025 0.5
0.30-0.39 (n=128) 36 (28) 1.56 (1.08-2.26) 0.018 0.14
0.20-0.29 (n=102) 33 (32) 1.90 (1.27-2.82) 0.002 0.077
0.10-0.19 (n=71) 14 (20) 1.02 (0.57-1.80) 0.96 0.84
<0.10 (n=31) 7 (23) 1.20 (0.52-2.77) 0.67 0.81
AUC 0.54 [0.51 ? 0.56]
Positive predictive value: 25.5%
Negative predictive value: 81.6%](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-39-320.jpg)
![周術期の尿量と術後AKI (?臓?術)
41
Song Y, et al. Medicine (Baltimore). 2016 May;95(22):e3757. PMID: 27258505 .
Retrospective cohort, ?臓外科696症例 (術後AKI 37%), CPB中の尿量と術後AKIの関連を検証
CPB中UO< 4 mL/kg/hr
術後AKIリスクが増加
CPB UO< 4 mL/kg/hr CPB UO ≥4 mL/kg/hr
UO 4 mL/kg/hr
CPB Urine Output mL/kg/hr
Variables No AKI (n=439) AKI (n=257) P
Type of surgery
Mitral valve surgery only 201 (46) 136 (53) 0.69
Aortic valve surgery only 141 (32) 89 (35) 0.497
Double valve operation 42 (10) 52 (20) <.001
Coronary valve
operation
31 (7) 27 (11) 0.113
Aorta surgery 17 (4) 15 (6) 0.448
Adult congenital 21 (5) 15 (6) 0.545
Transplantation 1 (0) 3 (1) 0.145
Others 28 (6) 24 (9) 0.152
CPB time, min 105 [80–135] 127 [91–161] <0.001](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-41-320.jpg)
![周術期の尿量と術後AKI (?臓?術)
42
Pet?j? L, et al. J Cardiothorac Vasc Anesth. 2017 Jun;31(3):827-836. PMID: 27856153 .
Single-center, prospective cohort, ?臓外科術後638症例, 術後AKIと予後を検証
AKI: 28.7% (KDIGO: 1; 18.8%, 2;6.1%, 3; 3.8%), No AKI: 71.3%
術後AKI
UO+SCrで診断された?が死亡リスク?い HR: 4.9 [2.4-9.9]
術後AKIリスクファクター
Multivariable
Odds Ratio (95% CI)
p Value
Age,years 1.02(1.00-1.04) 0.027
Body mass index 430 kg/m2, yes/no 2.99 (1.87-4.78) 0.001
Chronic kidney disease, yes/no 4.08 (2.22-7.50) 0.001
Peripheralvascular disease, yes/no 1.95 (1.10-3.45) 0.023
Urgent/emergency versus elective surgery 1.76 (1.11-2.78) 0.017
Lowest hematocrit, % 0.95(0.92-0.99) 0.009
Vasopressor load, ?g/kg/min 3.72 (1.50-9.25) 0.005
Inodilator therapy, yes/no 2.49 (1.54-4.02) 0.001
Furosemide in intensive care unit, yes/no 3.84(2.47-5.99) 0.001
Multivariable Regression Analysis of Covariates Predicting AKI During First 5 Postoperative Days](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-42-320.jpg)



![尿量をターゲットにした循環管理
46
van der Zee EN, et al. BMC Anesthesiol. 2017 Feb 10;17(1):22.
Systematic Review. PMID: 28187752
尿量をターゲットにした循環管理が死亡割合に関連するかを検証, Systematic review, 36 RCT
CFM: Conventional Fluid Therapy
Mortality risk OR [95% CI] I2
Not Targeting UO in both protocol (13 RCT) 0.87 [0.59 - 1.27, p=0.94] 20%
Targeting UO only in CFM (7 RCT) 1.09 [0.59 – 2.00, p=0.91] 4.7%
Targeting UO in both protocol (16 RCT) 0.77 [0.59 - 1.01, p=0.47] 35.6%
Total (36 RCT) 0.825 [0.684 - 0.995, p=0.95] 28%
7RCTでGDT群で尿量を指標にしていなかったが、
死亡リスクは変わらなかった
尿量測定の30?死亡リスク
死亡との関連は?せず
尿量を輸液管理の指標として死亡割合との関連を直接検証した
研究はなかった
GDT vs. CFMの研究
尿量測定と30?死亡割合を提?している研究を選択](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-46-320.jpg)

















![IUC合併症
73
Saint S, et al. JAMA Intern Med. 2018 Aug 1;178(8):1078-1085. PMID: 29971436;
PMCID: PMC6143107.
Infectious Complication
Non-Infectious
Complication
Variable IRR (95% CI) P Value IRR (95% CI) P Value
Urinary Catheter Duration
3 days or Less 1 [Reference] 1 [Reference]
More than 3 days 1.38 (1.03-1.84) 0.03 1.27 (1.17-1.37) <.001
Other Factor, Sex; Reason; AUA Symptom Index Score; Age
Percentage of 2076 Patients Reporting Complications After IUC
Multivariable Linear Regression Model to Predict Infectious and Noninfectious Urethral
Catheter Complications
3?以上の尿カテ挿?は
合併症のリスク
?感染性合併症
感染症の5倍以上
Total [55.4%]
Woman [47.3%]
Men [58.6%] P < .001
Total [10.5%]
Women [15.5%]
Men [ 8.6%] P < .001](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-73-320.jpg)
![?感染性IUC合併症
74
Hollingsworth JM, et al. Meta-analysis,37studies, 2868 patients
合併症 頻度 95% CI
尿漏れ 10.6% [2.4 to 17.7]
尿道狭窄(Higher quality studies) 16.7% [13.0 to 20.8]
(Low quality studies) 3.4% [1.0 to 7.0]
血尿 4.7% [0.0 to 10.0]
事故抜去 4.0% [0.0 to 8.6]
留置中 !"#$%&' (
感染性合併症 15.3%
?感染性合併症 70.2%
痛み?は不快感 54.5%
切迫感や膀胱の痙攣 34.7%
?尿 27.4%
?膚障害 19.4%
その他の合併症
社会活動の制限 43.9%
?常?活の制限 39.5%
抜去後 (N=2034) %
尿漏れ 20.3%
排尿開始または尿?め困難 19.5%
排尿時痛または灼熱感 17.4%
排尿困難 12.1%
尿の噴き出し 9.2%
?膚障害 6.6%
挿?部の出? 4.6%
Saint S, et al. Prospective cohort, 4 US Hospital, 2076 patients with IUC.内科/外科急性期病棟, ICU
18歳以上、参加者に聴取し3?以内に尿カテ留置した患者、留置から14??と30??詳細聴取
?感染性合併症 約7割
Hollingsworth JM, et al. Ann Intern Med. 2013 Sep 17;159(6):401-10. PMID: 24042368.
Saint S, et al. JAMA Intern Med. 2018 Aug 1;178(8):1078-1085. PMID: 29971436; PMCID: PMC6143107.](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-74-320.jpg)
![Retrospective cohort, 慈恵医?葛飾医療センター, 低侵襲?術患者5112例, 術後の意識変容を解析。診察記録からせん妄と思われる状態を抽出。
Inverse Probability Weightingで調整
意識変容リスク
Fukushima T, et al. Ann Med Surg (Lond). 2021 Mar 6;64:102186. PMID: 33747493
IUCとせん妄
All Patients Inverse Propensity-Weighted Patients
Control IUC ASD Control IUC ASD
Number of patients, N (%) 2,249 2,863 3,080.94 2,863.00
Age, y 48 [36 - 63] 60 [44 - 72] 0.477 60 [47 - 71] 60 [44 - 72] 0.024
Surgery type, N (%) 0.498 0.089
General surgery*
, N (%) 633 (28.1) 1,283 (44.8) 1,393.3 (45.2) 1,283.0 (44.8)
ENT surgery?
, N (%) 820 (36.5) 498 (17.4) 457.3 (14.8) 498.0 (17.4)
Orthopedic surgery?
, N (%) 724 (32.2) 896 (31.3) 979.3 (31.8) 896.0 (31.3)
Others, N (%) 72 (3.2) 186 (6.5) 251.1 (8.1) 186.0 (6.5)
Age >65 y, Male,BMI >30 kg/m2,Surgery type, Nerve block, Fentanyl use
Odds ratio [95% CI]
All patients P-value IPW P-value
Urinary catheter 2.08[1.44 - 3.03] <0.001 1.97 [1.50 - 2.59] <0.001
Inverse Propensity-Weighted Patients
Control IUC ASD P-value
N 3,080.94 2,863.00
AMS or UTI 98.3 (3.2) 143.0 (5.0) 0.091 0.397
AMS, Altered Mental Status
尿カテ挿?
↓
意識変容のリスク因?](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-78-320.jpg)















![UTI減少プロジェクト
101
Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619.
プログラムによりUTI, IUC減少
UTI: 2.40 à 2.05 infections/1000 catheter-days, incidence rate ratio (IRR), 0.86 [0.76 to 0.96; P = 0.009]
Non-ICU (N=553)
Variable IRR (95% CI) P Value IRR (95% CI) P Value
UTI Catheter Use
Time 0.68 (0.56–0.82) <0.001 0.93 (0.90–0.96) <0.001
Teaching hospital 1.76 (1.03–3.01) 0.04 0.96 (0.73–1.26) 0.77
Rural hospital 0.90 (0.66–1.23) 0.51 0.89 (0.78–1.01) 0.07
CAH 2.36 (1.65–3.37) <0.001 0.95 (0.82–1.10) 0.47
Hospital size
(per 100-bed increase)
0.97 (0.90–1.05) 0.45 0.98 (0.95–1.02) 0.38
UTI
2.28 à 1.54 infections/1000
catheter-days,
IRR, 0.68 [0.56-0.82, P<0.001]
IUC
20.1 à 18.8%
IRR, 0.93 [0.90-0.96, P<0.001]
CAH: Critical-access hospital
全体でUTI, 尿カテ使?率減少](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-101-320.jpg)
![UTI減少プロジェクト
102
Saint S, et al. N Engl J Med. 2016 Jun 2;374(22):2111-9. PMID:27248619.
UTI
2.48 à 2.50 infections/1000 catheter-days,
IRR, 1.01 [0.87-1.17, P=0.90]
IUC
62.8 à 61.9%
IRR, 0.98 [0.96-1.01; P=0.15]
プログラムによりUTI, IUC減少
UTI: 2.40 à 2.05 infections/1000 catheter-days, incidence rate ratio (IRR), 0.86 [0.76 to 0.96; P = 0.009]
ICU (N=373)
Variable IRR (95% CI) P Value IRR (95% CI) P Value
UTI Catheter Use
Time 1.01 (0.87–1.17) 0.9 0.98 (0.96–1.01) 0.15
Teaching hospital 1.92 (1.32–2.80) 0.001 0.96 (0.88–1.06) 0.45
Rural hospital 0.83 (0.58–1.18) 0.3 0.85 (0.78–0.91) <0.001
CAH 2.60 (0.94–7.20) 0.07 0.81 (0.67–0.98) 0.03
Hospital size
(per 100-bed increase)
1.09 (1.02–1.16) 0.01 1.02 (1.01–1.04) 0.01
Rural Hospital, CAH à 尿カテ使?率 減少
CAH: Critical-access hospital](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-102-320.jpg)
![CAUTI教育プログラムの導?
104
Menegueti MG, et al. Medicine (Baltimore). 2019 Feb;98(8):e14417. PMID: 30813142
ブラジル南東部にある公?3次医療機関の9床の?般集中治療室, CAUTI予防プログラム導?後のCAUTI発?率とIUC使?率を検証
1st ; 2005–2006, CAUTIがアウトブレイク, プロトコール導?期 2nd ; 2007–2010, CDCガイドラインに基づく年2回のトレーニング
3rd ; 2011–2014, チェックリスト導? + 年2回トレーニング 4th ; 2015–2016 年2回のトレーニングを年1回新?トレーニングに変更
1st 2nd 3rd 4th
CAUTI mean incidence rate,
entire phase
(No. of episodes/1000 catheter-days [SD])
14.9
(7.5)
7.3
(5.0)
3. 8
(5.7)
1.1
(3.0)
CAUTI
導?前の 7% 程度まで減少](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-104-320.jpg)
![施設ガイドライン作成と救急室でのIUC
106
Fakih MG, et al. Acad Emerg Med. 2010 Mar;17(3):337-40. PMID: 20370769..
before-after, single center, 769 tertiatry care hospital, 2517 patients
施設ガイドライン作成による救急室でのIUC減少頻度を評価
Compliant with guidelines, n = 203 (63.0%) N (%)
Non?intensive care 6 L /min oxygen 40 (12.4)
Output monitoring in intensive care 39 (12.1)
Emergent pelvic ultrasound 33 (10.2)
Intubated 32 (9.9)
Neurogenic bladder 14 (4.3)
Emergency surgery 12 (3.7)
Urinary obstruction 10 (3.1)
Unresponsive 7 (2.2)
Acute hip fracture 6 (1.9)
Urologic procedures 4 (1.2)
Acute mental status changes with agitation 4 (1.2)
Stage 3 or 4 sacral decubitus ulcers with incontinence 1 (0.3)
Hospice or palliative care 1 (0.3)
Noncompliant with guidelines, n = 119 (37.0%)
No clear reason 64 (19.9)
Oxygen supplementation <6 L ? min 26 (8.1)
Dementia 16 (5.0)
Urine specimen collection 5 (1.6)
Incontinence 3 (0.9)
Patient request 3 (0.9)
Output monitoring outside intensive care 2 (0.6)
UC utilization 14.9% à 10.6% OR = 1.48, [95% CI 1.16-1.90, p = 0.002]
ガイドライン非遵守IUCの理由
理由なしが約20%](https://image.slidesharecdn.com/20211214indwellingurinarycatheter-211223120131/85/Indwelling-Urinary-Catheter-106-320.jpg)