4release
- 1. Drug release from Nanoparticles
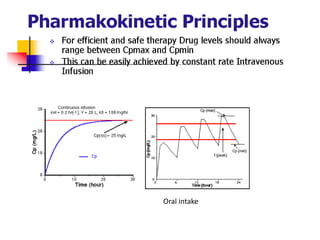
- 3. Oral intake
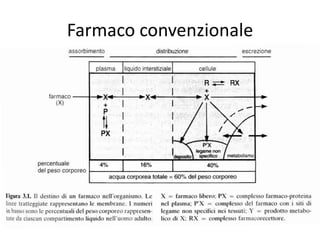
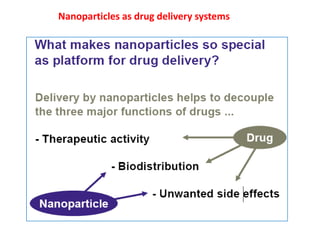
- 6. When a pharmaceutical agent is encapsulated within, or attached to, a polymer or lipid, drug safety and efficacy can be greatly improved and new therapies are possible. ? Drug targeting is concerned with modulation and control of the biodistribution of a drug based on a suitable delivery system. ? The biodistribution of the drug is not governed by the drug itself but by the delivery system. ? The biomedical design of the delivery system depends on the properties of the target in combination with those of the drug and the needs of the patient.
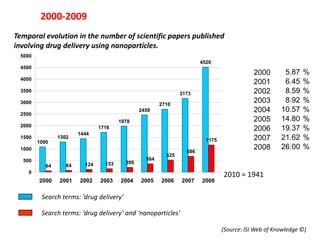
- 7. 2000-2009 Temporal evolution in the number of scientific papers published involving drug delivery using nanoparticles. 5000 4520 4500 2000 64 1090 2000 5.8764 % 4000 2001 84 1302 2001 6.4584 % 3500 2002 124 3173 1444 2002 8.59 % 124 3000 2003 153 2710 1716 2003 8.92 % 153 2004 2459 209 1978 2004 10.57 % 209 2500 2005 1978 364 2459 2005 14.80 % 364 2000 1716 2006 525 2710 2006 19.37 % 525 1444 1500 1302 2007 686 3173 1175 2007 21.62 % 686 1090 1000 2008 1175 4520 2008 26.00 % 1175 686 2009 364 154 525 446 446 2009 34.53 % 154 500 209 64 84 124 153 154 0 2010 = 1941 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Search terms: ĄŪdrug deliveryĄŊ Search terms: ĄŪdrug deliveryĄŊ and ĄŪnanoparticlesĄŊ (Source: ISI Web of Knowledge ?)
- 8. Nanoparticles as drug delivery systems
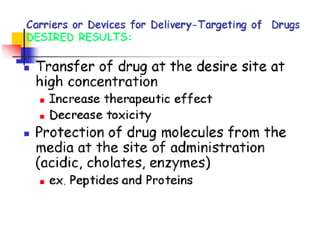
- 9. Potential advantages of improved drug delivery: Ability to target specific locations in the body ? Reduction of the quantity of drug needed to attain a particular concentration in the vicinity of the target; ? Decreased number of dosages and possibly less invasive dosing ? Reduction of harmful side effects due to targeted delivery (reduced concentration of the drug at non-target sites); Facilitation of drug administration for pharmaceuticals with short in vivo half-lives (for example peptides and proteins). Advantages must be weighed against the following concerns in the development of each particular drug-delivery system: 1. toxicity of the materials (or their degradation products) from which the drug is released, or other safety issues such as unwanted rapid release of the drug (dose dumping); 2. discomfort caused by the system itself or the means of insertion; 3. expense of the system due to the drug encapsulation materials or the manufacturing process.
- 10. METHODS OF DRUG DELIVERY ?epicutaneous (application onto the skin). the active substance diffuses through skin in a transdermal route. ?intradermal, (into the skin itself) is used for skin testing some allergens, and also for mantoux ?subcutaneous (under the skin), e.g. insulin ?nasal administration (through the nose) ?intravenous (into a vein), e.g. many drugs, total parenteral nutrition ?intraarterial (into an artery), ?intramuscular (into a muscle), e.g. many vaccines, antibiotics, and long-term psychoactive agents. ?intracardiac (into the heart), e.g. adrenaline during cardiopulmonary resuscitation (no longer commonly performed) ?intraosseous infusion (into the bone marrow) is, in effect, an indirect intravenous access because the bone marrow drains directly into the venous system ?intrathecal (into the spinal canal) is most commonly used for spinal anesthesia and chemotherapy ?intraperitoneal, (infusion or injection into the peritoneum) e.g. peritoneal dialysis ?Intravesical infusion is into the urinary bladder. ?intravitreal, through the eye
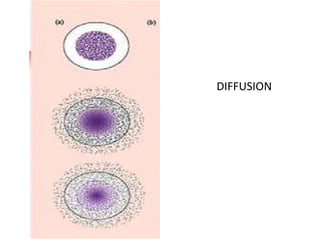
- 11. NP drug delivery systems Possible mechanisms by which drugs are released: 1. Diffusion of the drug species from or through the system. 2. A chemical or enzymatic reaction leading to degradation of the system, or cleavage of the drug from the system. 3. Water activation, either through osmosis or swelling of the system. Rosen & Abribad, Nature Reviews 2005
- 12. DIFFUSION
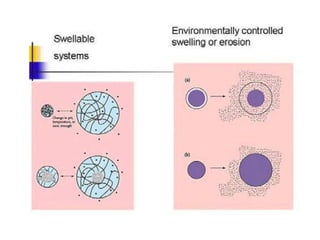
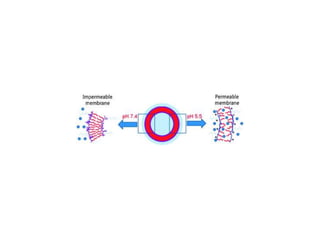
- 14. Ways of controlling drug release locally: Smart Stimuli-responsive NPs Stimuli-responsive NPs show a sharp change in properties upon a small or modest variations of the environmental conditions such as temperature, light, salt concentration or pH. Different organs, tissues and cellular compartments may have large differences in pH, which is considered the most suitable stimulus. This behavior can be used for the preparation of so-called ĄŪsmartĄŊ drug delivery systems, which mimic biological response behavior to a certain extent. Schmalijohann D., Adv. Drug Deliv. Rev, 58 2006
- 15. Ways of controlling drug release locally ? pH ? Light ? Thermally ? Ultrasound ? Magnetically ? Enzyme-induced
- 16. pH in living systems[ Compartment pH Gastric acid 1 Lysosomes 4.5 Granules of chromaffin cells 5.5 Human skin 5.5 Urine 6.0 Cytosol 7.2 Cerebrospinal fluid (CSF) 7.5 Blood 7.34ĻC7.45 Mitochondrial matrix 7.5 Pancreas secretions 8.1 Solid tumours 6.5
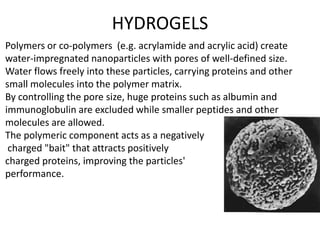
- 17. HYDROGELS Polymers or co-polymers (e.g. acrylamide and acrylic acid) create water-impregnated nanoparticles with pores of well-defined size. Water flows freely into these particles, carrying proteins and other small molecules into the polymer matrix. By controlling the pore size, huge proteins such as albumin and immunoglobulin are excluded while smaller peptides and other molecules are allowed. The polymeric component acts as a negatively charged "bait" that attracts positively charged proteins, improving the particles' performance.
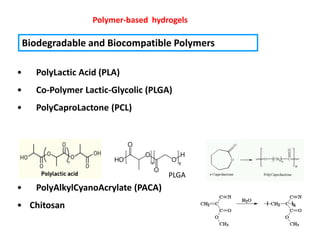
- 18. Polymer-based hydrogels Biodegradable and Biocompatible Polymers ? PolyLactic Acid (PLA) ? Co-Polymer Lactic-Glycolic (PLGA) ? PolyCaproLactone (PCL) PLGA ? PolyAlkylCyanoAcrylate (PACA) ? Chitosan
- 19. Hydrogels are three dimensional networks of polymers
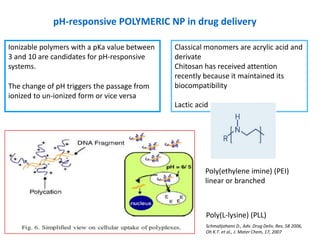
- 20. pH-responsive POLYMERIC NP in drug delivery Ionizable polymers with a pKa value between Classical monomers are acrylic acid and 3 and 10 are candidates for pH-responsive derivate systems. Chitosan has received attention recently because it maintained its The change of pH triggers the passage from biocompatibility ionized to un-ionized form or vice versa Lactic acid Poly(ethylene imine) (PEI) linear or branched Poly(L-lysine) (PLL) Schmalijohann D., Adv. Drug Deliv. Rev, 58 2006, Oh K.T. et al., J. Mater Chem, 17, 2007
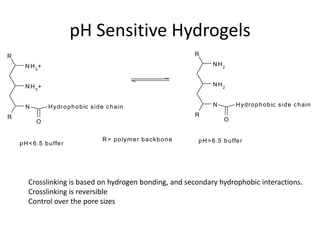
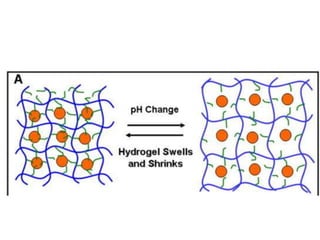
- 21. pH Sensitive Hydrogels R R N H3 + N H2 N H3 + N H2 N H y dr o p h o b ic s id e c h a in N H y dr o p h o b ic s id e c h a in R R O O R = p o lym e r b a ckb o n e p H > 6 .5 b u ffe r p H < 6 .5 b u ffe r Crosslinking is based on hydrogen bonding, and secondary hydrophobic interactions. Crosslinking is reversible Control over the pore sizes
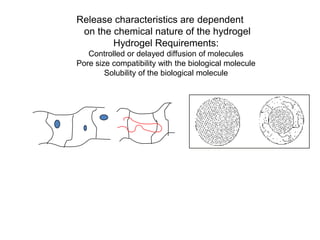
- 23. Release characteristics are dependent on the chemical nature of the hydrogel Hydrogel Requirements: Controlled or delayed diffusion of molecules Pore size compatibility with the biological molecule Solubility of the biological molecule
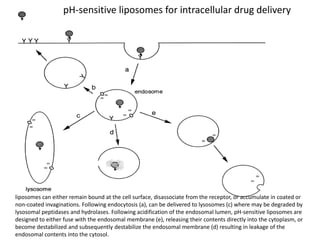
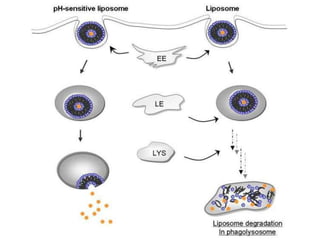
- 26. pH-sensitive liposomes for intracellular drug delivery liposomes can either remain bound at the cell surface, disassociate from the receptor, or accumulate in coated or non-coated invaginations. Following endocytosis (a), can be delivered to lysosomes (c) where may be degraded by lysosomal peptidases and hydrolases. Following acidification of the endosomal lumen, pH-sensitive liposomes are designed to either fuse with the endosomal membrane (e), releasing their contents directly into the cytoplasm, or become destabilized and subsequently destabilize the endosomal membrane (d) resulting in leakage of the endosomal contents into the cytosol.
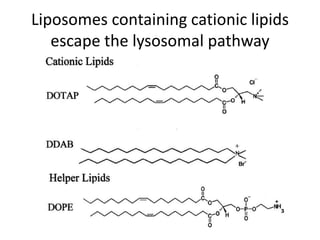
- 27. Liposomes containing cationic lipids escape the lysosomal pathway
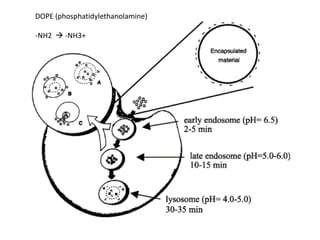
- 29. DOPE (phosphatidylethanolamine) -NH2 ? -NH3+
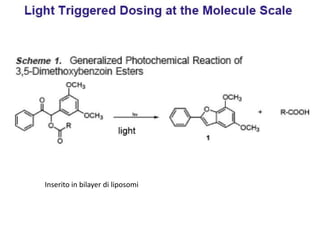
- 30. Inserito in bilayer di liposomi
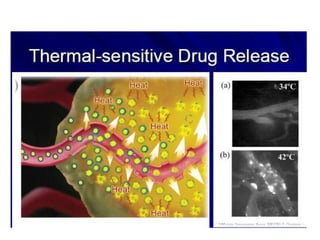
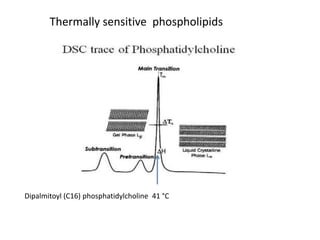
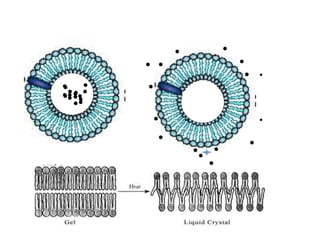
- 32. Thermally sensitive phospholipids Dipalmitoyl (C16) phosphatidylcholine 41 ĄãC
- 33. ? ? ? ? ? ? ? ?? ?? ? ? ???? ??? ??? ?? ? ? ? ? ?? ? 37ĄãC ? 41ĄãC
- 34. Thermo-responsive POLYMERS in drug delivery Temperature-responsive polymers and hydrogels exhibit a volume phase transition at a certain temperature, which causes a sudden change in the solvation state. Upper critical solution Lower critical solution temperature (UCST) temperature (LCST) PNIPAM in drug delivery Poly-N-IsoPropylAcrilAmide (PNIPAM) is the most prominent candidate as thermo- responsive polymer. PNIPAM copolymers have been mainly studied for the oral delivery of calcitonin and insulin. Schmalijohann D., Adv. Drug Deliv. Rev, 58 2006
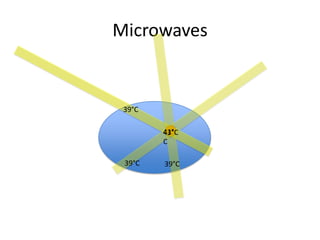
- 35. Microwaves 39ĄãC 41Ąã 43ĄãC C 39ĄãC 39ĄãC
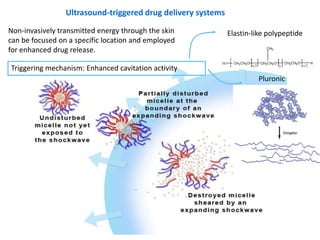
- 36. Ultrasound-triggered drug delivery systems Non-invasively transmitted energy through the skin Elastin-like polypeptide can be focused on a specific location and employed for enhanced drug release. Triggering mechanism: Enhanced cavitation activity Pluronic
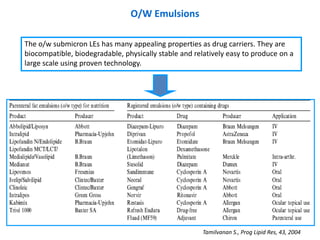
- 37. O/W Emulsions The o/w submicron LEs has many appealing properties as drug carriers. They are biocompatible, biodegradable, physically stable and relatively easy to produce on a large scale using proven technology. Tamilvanan S., Prog Lipid Res, 43, 2004
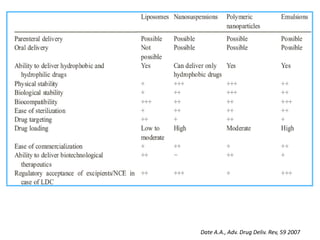
- 38. Date A.A., Adv. Drug Deliv. Rev, 59 2007