Irwa Presentation 2009
- 1. Ėý
- 2. OVERVIEW Chlorine Dioxide use & attributes Review of ClO 2 generation used in drinking water applications: 3-Chem (12.5% Bleach +25% NaClO 2 +15%HCL Acid) 2-Chem (Cl 2 gas + 25%NaClO 2 ) Electrochemical (25%NaClO 2 + H 2 O) Results from ClO 2 pre-disinfection applications
- 3. ClO2 Molecular Attributes Lowest Oxidation-Reduction Potential Selective Oxidizer Which Does Not React With Background Organics Effective For the Removal of Bio-Film Works Over a Broad pH Range Readily Reacts With Iron & Manganese
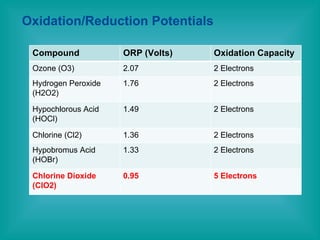
- 4. Oxidation/Reduction Potentials Compound ORP (Volts) Oxidation Capacity Ozone (O3) 2.07 2 Electrons Hydrogen Peroxide (H2O2) 1.76 2 Electrons Hypochlorous Acid (HOCl) 1.49 2 Electrons Chlorine (Cl2) 1.36 2 Electrons Hypobromus Acid (HOBr) 1.33 2 Electrons Chlorine Dioxide (ClO2) 0.95 5 Electrons
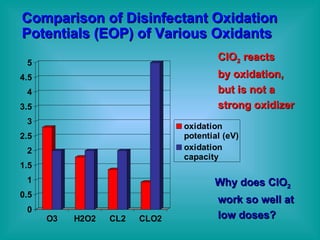
- 5. Comparison of Disinfectant Oxidation Potentials (EOP) of Various Oxidants ClO 2 reacts by oxidation, but is not a strong oxidizer Why does ClO 2 work so well at low doses?
- 6. Does Not React With Background Organics Does Not React With Aldehydes Ethers Ammonia Fats Acids Glycols Alkanes Ketones Alkynes Polysaccharides Alcohols Saccharides Amines Unsaturated Fatty Acids Carbohydrates Unsaturated Aromatics
- 7. ClO 2 Efficacy from Chemical Selectivity HOCl, H 2 O 2 and O 3 Are Indiscriminant Oxidants React with nearly any organic or inorganic species present Consumed by useless side-reactions Chlorine Dioxide Reacts Selectively Reacts rapidly with sulfides (S 2- ) Reacts rapidly with aromatic hydroxides (phenols) ClO 2 less effected by organic contamination
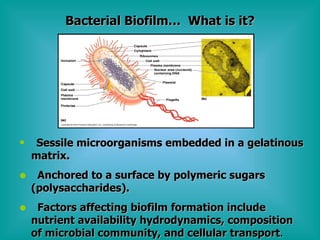
- 8. Bacterial BiofilmâĶ What is it? Sessile microorganisms embedded in a gelatinous matrix. Anchored to a surface by polymeric sugars (polysaccharides). Factors affecting biofilm formation include nutrient availability hydrodynamics, composition of microbial community, and cellular transport .
- 9. Aerobic Bacteria Slimers - Pseudomonads, Mucoids Spores - Bacillis Subtilis Fecal - Enterobacter Ubiquitous - Anabena, Asterionella Anaerobic Bacteria Sulfate Reducing Bacteria â Desulfovibrio Iron Reducing Bacteria â Gallionella Protozoa Consume bacteria Types & Kinds of Micro-organisms
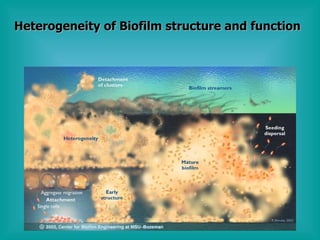
- 10. Heterogeneity of Biofilm structure and function
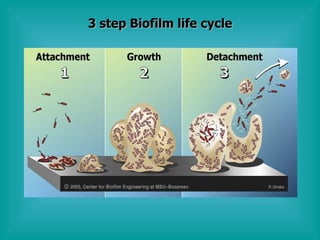
- 11. Interpretation of Biofilm Diversity Aerobic and anaerobic species capable of co- existing. Proliferating and dormant species co-exist. Single layer to 3 dimensional structures. Unicellular to multi-cellular. Biofilm remediation indicates that a weaker disinfectant, (if it penetrates) will easily outperform a strong disinfectant that fails to penetrate
- 12. 3 step Biofilm life cycle
- 13. Good planktonic control does not always correlate with good biofilm control A biofilm under low continuous stress may persist and continue to grow slowly Typically monitor planktonic counts, leaving us with a false sense of security High stress can âshockâ biofilm off walls, resulting in more complete disinfection Therefore, best practice for biofilm elimination is not continuous feeding of disinfectant, but shock treatment of the films Biofilm vs. Planktonic Control
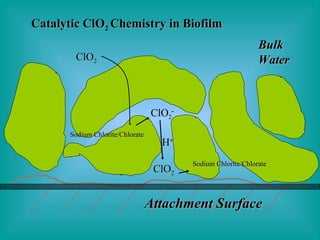
- 14. Attachment Surface Bulk Water ClO 2 S-S ClO 2 - ClO 2 H + Catalytic ClO 2 Chemistry in Biofilm Sodium Chlorite/Chlorate Sodium Chlorite/Chlorate
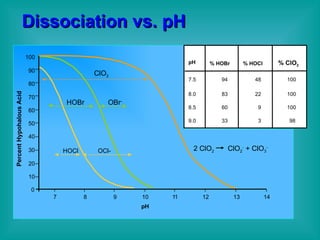
- 15. Dissociation vs. pH pH 100 90 80 70 60 50 40 30 20 10 0 7 8 9 10 1 1 12 13 14 HOBr OBr - HOCl OCl- Percent Hypohalous Acid ClO 2 pH % HOBr % HOCl % ClO 2 7.5 8.0 8.5 9.0 94 83 60 33 48 22 9 3 100 100 100 98 2 ClO 2 ClO 2 - + ClO 3 -
- 16. Chlorine Dioxide-Manganese Reaction 2Cl0 2 + Mn 2+ +2H 2 O ï 2Cl0 2 - + MnO 2 + 4H + Theoretical Consumption 2.45 mg of Cl0 2 per mg of Mn 2+
- 17. Chlorine Dioxide-Iron Reaction Cl0 2 + Fe 2+ ï Fe 3+ + Cl0 2 - Fe 3+ + 3 OH - ï Fe(OH) 3 Theoretical Consumption 1.2 mg of Cl0 2 per mg of Fe 2+
- 18. Dual Wet Chem Generators Chlorine Gas Sodium Chlorite (dry or liquid) Three Wet Chem Generators 12.5% Sodium Hypo-chlorite 15% Hydrochloric Acid 25% Sodium Chlorite Traditional ClO 2 Generation Methods
- 19. Traditional ClO 2 Generation Methods Cumbersome Increased chemical handling and management Difficult to optimize CLO 2 conversation CLO 2 external batch tank required Complicated Difficult to operate, troubleshoot and repair Impure Product need to over feed acid and bleach for a higher percent conversation increases more unwanted by-products
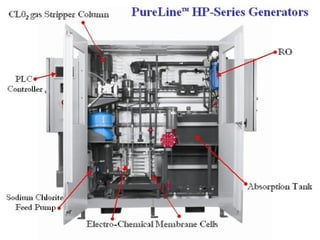
- 20. Electrochemical ClO 2 Generation SIMPLE Turnkey installation Fully automated Color touch screen PLC controller interface Flow paced feed SAFE single precursor technology (PureCide 25 TM solution) reliable operation multiple safety interlocks and alarm features PURE 99.5% pure CLO2 gas CLO 2 gas diluted with water into adsorption column
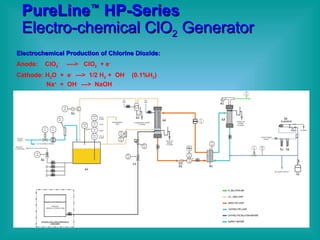
- 21. PureLine ÂŪ HP-Series Generators Electrochemical ClO 2 Production: Anode: ClO 2 - ï ClO 2 + e - + Na + Cathode: H 2 O + e - ï 1/2 H 2 + OH - Na + + OH - ï NaOH
- 22. PureLine âĒ HP-Series Electro-chemical ClO 2 Generator Electrochemical Production of Chlorine Dioxide: Anode: ClO 2 - ----> ClO 2 + e - Cathode: H 2 O + e - ---> 1/2 H 2 + OH - (0.1%H 2 ) Na + + OH - ---> NaOH
- 23. Ėý
- 24. Electrochemical CIO 2 Advantages Provides 99.5% Pure ClO 2 Single Precursor (PureCide 25 TM solution) On-Demand Feed Control No External Water Pretreatment Required ClO 2 Production Capacity Up To 80 lbs/day DCS Capable/Automated Feed Control
- 25. Electrochemical CIO 2 Operational Costs Electrical Consumption Per Pound of ClO 2 55 watts per lb. Chlorine Dioxide $0.085/KWH = $7.14 per day electrical usage PureCide 25 TM solution Consumption 6.6 lbs Per Pound of ClO 2
- 26. DBP Monitoring Greenville, Il: Testing Started in 1990 101 ppb TTHM (1 st Quarter Average) 47 ppb TTHM (2 nd Quarter Average) 123 ppb TTHM (3 rd Quarter Average) 107 ppb TTHM (4 th Quarter Average) What To Do? What are my choices?
- 27. Ways to Minimize DBPs Upgrade to Micro-Filtration Move Back the Cl2 Pre-Disinfection Point Pre-Disinfect with Ozone or UV Pre-Disinfect with Chlorine Dioxide
- 28. Ways to Minimize DBPs Microfiltration Very effective, non-chemical means to remove suspended particulates down to sub-micron levels Very expensive: ~ $8-$10 MM fully installed for 2.5 MM GPD Requires extensive maintenance
- 29. Ways to Minimize DBPs Move Back the Cl 2 Pre-Disinfection Point Reduction in CT- credits Increased corrosion if an increased Cl 2 dose is required for CT-credit pH sensitive
- 30. Ways to Minimize DBPs Pre-Disinfect with Ozone - Increases the potential for biodegradable organic matter, creating a requirement for a biologically active filter. Complex operation requires significant training and maintenance Capital costs estimated at $0.5 to $1 MM No THMs or HAAs, but potential for bromate formation (10 ppb MCL) Bromate formation occurs faster than pathogen inactivation, Bromate increase with increase of O 3 dosage feed. High efficacy at low dose Must be produced on-site Energy intensive No residual
- 31. Ways to Minimize DBPs Pre-Disinfect with Chlorine Dioxide More efficacy at a lower dose than Cl 2 (CT-values) Not sensitive to pH No THM or HAA production No bromate production Must be produced on-site Effective for taste and odor Effective for turbidity reduction Minimal capital cost expenditure
- 32. ClO 2 On-Site Generation Prefer the chemistry of ClO 2 , but are concerned about: storing and handling multiple chemical precursors purity of the ClO 2 generated monitoring and maintaining ClO 2 and NaClO 2 residuals below the MCLs reliability and simplicity of the generation and feed system
- 33. ClO 2 Regulatory Status MRDL of 1.0 mg/L â Chlorite/Chlorate Ion MCL of 0.8 mg/L â Chlorine Dioxide
- 34. Chlorine Dioxide, Chlorite, Chlorate Analytical Methods Compliance monitoring for measuring residual Chlorine Dioxide A) DPD Standard Method 4500-CLO 2 D B) Amperometric Titration Method II Standard Method 4500-CLO 2 E Compliance monitoring for measuring residual Chlorite/Chlorate Ion A) Amperometric Titration Standard Method 4500-CLO 2 E, B) Ion Chromatography EPA Method 300.0 & 300.1 Amperometric Titration may be used for routine daily monitoring of Chlorite at the entrance to the distribution system.
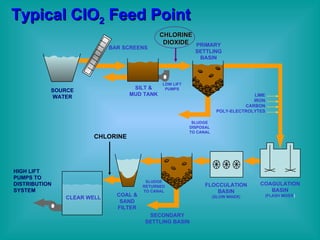
- 35. Typical ClO 2 Feed Point SOURCE WATER SILT & MUD TANK PRIMARY SETTLING BASIN SECONDARY SETTLING BASIN FLOCCULATION BASIN (SLOW MIXER) COAGULATION BASIN (FLASH MIXER ) CLEAR WELL COAL & SAND FILTER BAR SCREENS CHLORINE DIOXIDE LOW LIFT PUMPS SLUDGE DISPOSAL TO CANAL SLUDGE RETURNED TO CANAL CHLORINE HIGH LIFT PUMPS TO DISTRIBUTION SYSTEM LIME IRON CARBON POLY-ELECTROLYTES
- 36. Pure ClO 2 Results Use of pure ClO 2 for pre-disinfection provided several benefits: Improved disinfection Improved DBP reduction Improved turbidity reduction Improved taste and odor Improved safety
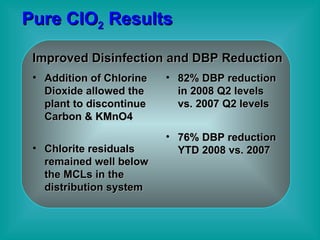
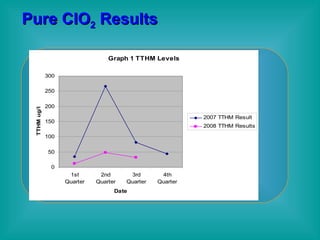
- 37. Pure ClO 2 Results Improved Disinfection and DBP Reduction Addition of Chlorine Dioxide allowed the plant to discontinue Carbon & KMnO4 Chlorite residuals remained well below the MCLs in the distribution system 82% DBP reduction in 2008 Q2 levels vs. 2007 Q2 levels 76% DBP reduction YTD 2008 vs. 2007
- 38. Pure ClO 2 Results
- 39. Chlorine âChlorinatesâ, Chlorine Dioxide âOxidizesâ HOCl + Organic ï Cl-Organic (THM/HAA/AOX) ClO 2 (O=Cl=O) + Organic ï O-Organic (-) O 3 (O-O-O) + Organic ï O-Organic (-)
- 40. Pure ClO 2 Results â Taste & Odor Taste & Odor greatly improved even with the discontinued use of Carbon & KMnO4 Oxidation of metals and organics to make more negatively charged particles that readily bond to the cationic coagulant â People in town were asking what had changed. The water tasted more like bottled water.â
- 41. Pure ClO 2 Results â Improved Safety Greenville, Il Cl 2 gas cylinder usage reduced by 50% Automated feed system provided operator-free operation KMnO4 & Carbon elimination resulted in reduced operator exposure to chemicals
- 42. Conclusions The City of Greenville was faced with unacceptably high concentrations of DBPs due to pre- and post-chlorination of surface water.
- 43. Conclusions ClO 2 chosen over alternatives: - increased CT-credits - improved DBP reduction - eliminated KnMnO4 & Carbon - improved operator safety - simplicity of feed
- 44. Conclusions Pre-disinfection with pure ClO 2 provided a very cost-effective means for dramatically reducing DBP levels while improving the microbial control, turbidity, taste and odor with a reduction in chemical use resulting in a 3.5 â 4 year ROI.
- 45. Conclusions Use of Pure CIO 2 Resulted in : increase in CT credits 82% TTHM reduction Q2 2008 vs. Q2 2007 76% TTHM reduction in 2008 vs. 2007 50% reduction in use of Cl2 gas Improved taste & odor
- 46. Questions? PureLine Treatment Systems www.pureline.com