Aas presentation
- 2. Atomic Absorption (AA) spectroscopy âĒ âĒ Atomic absorption spectroscopy is a quantitative method of analysis that is applicable to many metals and a few nonmetals. A few examples include: Al in blood serum Ca in blood serum, plants, soil, water Cu in alloys Cr in sea water Fe in plants âĒ âĒ âĒ Only a drop of sample needed The metals need not be removed from other components (AA is a highly selective technique) Sensitive in the ppm range (even ppb with the right equipment)
- 3. Principles of AAS âĒ âĒ When metals are exposed to heat, they absorb light. Each metal absorbs light at a characteristic frequency. For example: Metal Îŧ (nm) Zn 214 Fe 248 Cu 325 Ca 423 Na 589
- 4. âĒ âĒ âĒ The metal vapor absorbs energy from an external light source, and electrons jump from the ground to the excited states The ratio of the transmitted to incident light energy is directly proportional to the concentration of metal atoms present A calibration curve can thus be constructed [Concentration (ppm) vs. Absorbance]
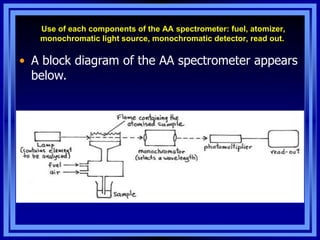
- 5. Use of each components of the AA spectrometer: fuel, atomizer, monochromatic light source, monochromatic detector, read out. âĒ A block diagram of the AA spectrometer appears below.
- 6. Overview of AA spectrometer. Light Source Sample Compartment Detector
- 7. âĒ âĒ âĒ The source of light is a lamp whose cathode is composed of the element being measured. Each analyzed element requires a different lamp. For example, a hollow cathode lamp for Aluminum (Al) is shown below
- 8. âĒ The cathode lamps are stored in a compartment inside the AA spectrometer. The specific lamp needed for a given metal analysis is rotated into position for a specific experiment.
- 9. âĒ âĒ âĒ The sample is made up, typically in water A flame is created, usually using ethyne & oxygen (fuel) The flame gases flowing into the burner create a suction that pulls the liquid into the small tube from the sample container. This liquid is transferred to the flame where the sample is atomized [mixing the sample with air to create fine droplets]. The metal atoms then absorb light from the source (cathode lamp).
- 10. Light beam Sample is vaporized in the flame. Aspirator tube sucks the sample into the flame in the sample compartment.
- 11. âĒ âĒ âĒ The light passes through a monochromater (a device used to select a particular wavelength of light for observation) The intensity of the light is fairly low, so a photomultiplier tube (PMT) is used to boost the signal intensity A detector (a special type of transducer) is used to generate voltage from the impingement of electrons generated by the photomultiplier tube
- 12. A typical photomultiplier tube
- 13. âĒ The read out specified by the user is displayed on the computer screen for each sample measured.
- 14. The resulting data can be presented in a variety of ways, but typically a print out is made.
- 15. Concentration of a solution from a calibration curve. âĒ âĒ âĒ AA can be used to identify the presence of an element (qualitative analysis), or the concentration of a metal (quantitative analysis) Quantitative analysis can be achieved by measuring the absorbance of a series of solutions of known concentration. A calibration curve and the equation for the line can be used to determine an unknown concentration based on its absorbance.
- 16. Disadvantages of AAS ïą only solutions can be analyzed ïą relatively large sample quantities required (1-2mL) ïą less sensitivity ïą problems with refractory elements
- 17. Advantages of AAS ï§ inexpensive (equipment, day-to-day running ïą high sample throughput ïą easy to use ïą high precision




![âĒ
âĒ
âĒ
The metal vapor absorbs energy from an
external light source, and electrons jump from
the ground to the excited states
The ratio of the transmitted to incident light
energy is directly proportional to the
concentration of metal atoms present
A calibration curve can thus be constructed
[Concentration (ppm) vs. Absorbance]](https://image.slidesharecdn.com/aaspresentation-140212033857-phpapp01/85/Aas-presentation-4-320.jpg)



![âĒ
âĒ
âĒ
The sample is made up, typically in water
A flame is created, usually using ethyne &
oxygen (fuel)
The flame gases flowing into the burner create a
suction that pulls the liquid into the small tube
from the sample container. This liquid is
transferred to the flame where the sample is
atomized [mixing the sample with air to create
fine droplets]. The metal atoms then absorb
light from the source (cathode lamp).](https://image.slidesharecdn.com/aaspresentation-140212033857-phpapp01/85/Aas-presentation-9-320.jpg)







