Aba ppt 9130
- 1. 1
- 2. UNIVERSITY OF AGRICULTURAL SCIENCES, RAICHUR Discovery, Occurance , Chemical structure of Abssiscic acid Gangadharayya S . Hiremath PG17AGR9130 Dept of seed science &Technology COA, Raichur 2
- 3. •Introduction • History • Chemical composition • Occurrence and distribution • Biosynthesis •Physiological roles CONTENTS 3
- 4. HISTORY •In 1940s, scientists isolated a substance from Sycamore leaves called Dormins. (Hemberg) •In the early 1960s, Eagles and Philip Wareing confirmed that application of a dormin to a bud would induce dormancy •F.T. Addicott (1963) discovered a substance stimulated abscission of cotton fruit. He named this substance as abscisin II • In 1964, it became evident that the three groups had discovered the same plant hormone. • Later on the name was changed to abscisic acid(ABA). 4
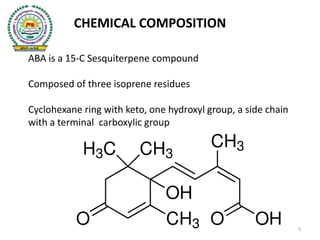
- 5. CHEMICAL COMPOSITION ABA is a 15-C Sesquiterpene compound Composed of three isoprene residues Cyclohexane ring with keto, one hydroxyl group, a side chain with a terminal carboxylic group 5
- 6. Synonyms: 1) ABA 2) Dormin 3) Absicin II Molecular Formula: C15H20O4 Molecular Weight: 264.32 g Appearance: White crystals Purity :98% Chemical name Abscisic acid: (2-cis,4-trans)-5-(1-Hydroxy-2,6,6- trimethyl-4-oxo-2-cyclohexen-1-yl)-3-methyl-2,4-pentadienoic acid 6
- 7. The orientation of carboxylic group at carbon 2 determine the cis and trans isomers of ABA Cis-Absscisic acid (biologically active) Trans-Absscisic acid (biologically inactive) Nearly all the naturally occurring ABA is in the cis form 7
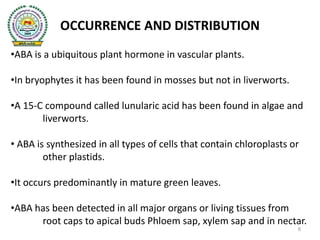
- 8. OCCURRENCE AND DISTRIBUTION •ABA is a ubiquitous plant hormone in vascular plants. •In bryophytes it has been found in mosses but not in liverworts. •A 15-C compound called lunularic acid has been found in algae and liverworts. • ABA is synthesized in all types of cells that contain chloroplasts or other plastids. •It occurs predominantly in mature green leaves. •ABA has been detected in all major organs or living tissues from root caps to apical buds Phloem sap, xylem sap and in nectar. 8
- 9. BIOSYNTHESIS Initial stages occur in the plastids, where isopentenyl diphosphate (IPP) is converted to the C40 xanthophyll Zeaxanthin Zeaxanthin is further modified to 9-cisneoxanthin, which is cleaved by the enzyme NCED (9-cis epoxycarotenoid dioxygenase) to form the C15 inhibitor, Xanthoxal Xanthoxal is then converted to ABA in the cytosol 9
- 10. 10
- 11. Mutants have been isolated that cause defects in the conversion of ABA aldehyde into ABA ÔÇß Flacca and Sitiens in Solanum lycopersicum ÔÇß nar2a in Hordeum vulgare ÔÇß Aba3 and aao3 in Arabidopsis 11
- 12. • Promotes stomatal closing. •Induces bud dormancy and seed dormancy. • Promotes desiccation tolerance in the embryo. •Inhibits precocious germination and vivipary •Promotes root growth and inhibits shoot growth in stressed water condition •Leaf Senescence PHYSIOLOGICAL ROLE OF ABA 12
- 13. 13