Acid base disorders
- 2. âŦØĻØģŲ ا اŲØąØŲ Ų⎠âŦاŲØąØŲŲ ⎠âŦâ ØąØĻ اØīØąØ ŲŲ⎠âŦØĩØŊØąŲ⎠âŦŲŲØģØą ŲŲ ØĢŲ ØąŲ⎠âŦŲاØŲŲ ØđŲØŊØĐ Ų Ų⎠âŦŲØģاŲŲâŽ
- 3. APPROACH TO ACID-BASE DISORDERS
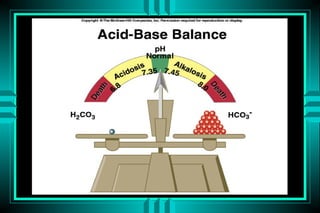
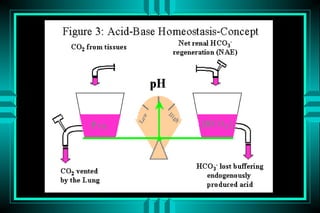
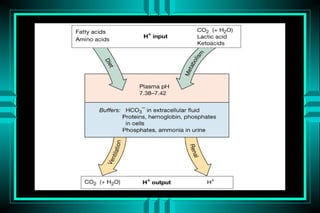
- 4. Definitions Acid: a substance that may donate protons (hydrogen ions) Base: a substance that may receive protons pH: the negative logarithm of protons concentration Strong acids vs. weak acids Volatile (Co2) vs. nonvolatile acids Buffers
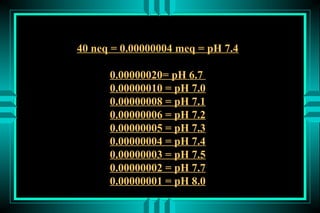
- 8. 40 neq = 0.00000004 meq = pH 7.4 0.00000020= pH 6.7 0.00000010 = pH 7.0 0.00000008 = pH 7.1 0.00000006 = pH 7.2 0.00000005 = pH 7.3 0.00000004 = pH 7.4 0.00000003 = pH 7.5 0.00000002 = pH 7.7 0.00000001 = pH 8.0
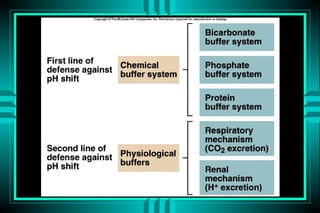
- 13. Buffering System
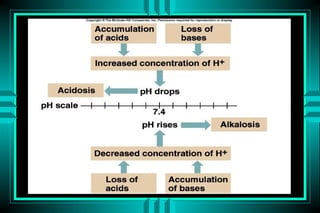
- 14. ACID-BASE DISORDERS DEFINITIONS ï· ACIDEMIA VS ALKALEMIA ï· ACIDOSIS VS ALKALOSIS ï· RESPIRATORY VS METABOLIC ï· COMPENSATORY RESPONSES ï· SIMPLE (SINGLE) VS MIXED
- 15. ACID-BASE DISORDERS DIAGNOSIS BASED ON: SUGGESTIVE HISTORY SUGGESTIVE PHYSICAL EXAM SUGGESTIVE CO2, K+, CL- SUGGESTIVE pH, PCO2, HCO3-, AG
- 16. APPROACH TO THE DIAGNOSIS OF ACID-BASE DISORDERS Suspicious clinical or lab findings Identify the major Acid-base disorder Determine if it is simple or mixed Establish the cause of the disorder Direct treatment to the underlying cause unless the pH is in a dangerous range (7.10 < or > 7.60)
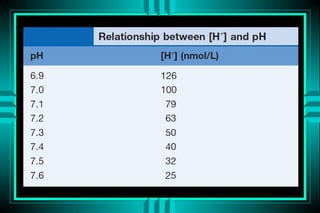
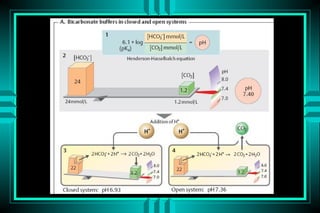
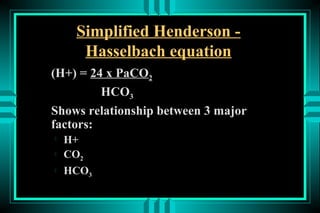
- 17. ACID-BASE DISORDERS FORMULAS: HENDERSON-HASSELBALCH ïŽ pH = pK + log ([HCO3-]/[0.03* PCO2]) ïŽ pH = 6.10 + log (24/0.03*40) = 7.40 MODIFIED HENDERSON ïŽ [H+] = 24* PCO2/[HCO3-] ïŽ [H+] = 24* (40/24) = 40 neq/L (pH=7.4)
- 18. Simplified Henderson - Hasselbach equation (H+) = 24 x PaCO2 HCO3 Shows relationship between 3 major factors: ïŽ H+ ïŽ CO2 ïŽ HCO3
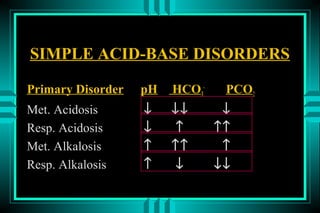
- 19. SIMPLE ACID-BASE DISORDERS Primary Disorder pH HCO3- PCO2 Met. Acidosis â ââ â Resp. Acidosis â â ââ Met. Alkalosis â ââ â Resp. Alkalosis â â ââ
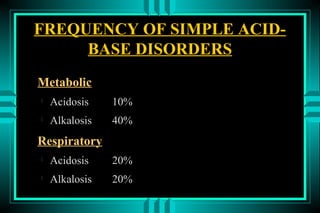
- 21. FREQUENCY OF SIMPLE ACID- BASE DISORDERS Metabolic ïŽ Acidosis 10% ïŽ Alkalosis 40% Respiratory ïŽ Acidosis 20% ïŽ Alkalosis 20%
- 22. Consequences of acidosis vs. alkalosis Impaired cardiac contractility Arteriolar dilation Venoconstriction Centralization of blood volume Increased pulmonary Arteriolar constriction vascular resistance Reduced coronary blood flow Cardiovascular Decreased cardiac output Reduced anginal threshold Decreased systemic BP Decreased threshold for Decreased hepatorenal cardiac arrhythmias blood flow Decreased threshold for cardiac arrhythmias Attenuation of responsiveness to catecholamines
- 23. Insulin resistance Stimulation of anaerobic Inhibition of anaerobic glycolysis glycolysis Formation of organic acids Reduction in ATP Decreased oxyhemoglobin Metabolic synthesis dissociation Hyperkalemia Decreased ionized Ca Protein degradation Hypokalemia Bone demineralization Hypomagnesemia (chronic) Hypophosphatemia Tetany Inhibition of metabolism Seizures and cell-volume Neurologic Lethargy regulation Delirium Obtundation and coma Stupor Compensatory Compensatory hyperventilation with Respiratory hypoventilation with possible respiratory hypercapnia and hypoxemia muscle fatigue
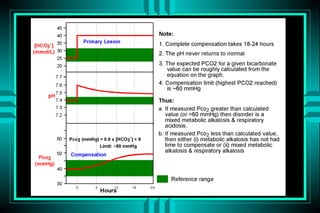
- 24. RESPONSE TO SIMPLE ACID-BASE DISORDERS Disturbance Equation Interval Level Met. Ac. 1 = 1.2 12-24 hr 10 Met. Al. 1 = 0.7 24-36 hr 55 Ac. Resp. Ac. 1 = 0.1 5-10 min 43 Ch. Resp. Ac. 1 = 0.3 72-120 hr 45 Ac. Resp. Al. 1 = 0.2 5-10 min 18 Ch. Resp. Al. 1 = 0.4 48-72 hr 13
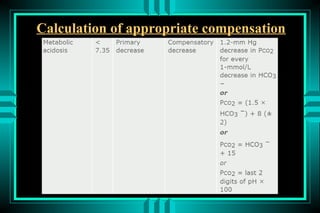
- 25. Calculation of appropriate compensation
- 29. Or In metabolic disorders add 15 to HCO3: Example: if HCO3=10 + 15 = 25 then the PCO2 should be 25, and the last two digits of pH 25 ïŽ pH=7.25 ïŽ HCO3=10 ïŽ PCO2=25
- 30. Normal Anion Gap AG = Na â (HCO3+Cl) = 8-12 A-10 HCO3- PaCO2 40 25 pH 7.40 Na 140 CL 105
- 31. METABOLIC ACIDOSIS AG = 10 AG = 10 AG = 25 HCO3 =15 HCO3 =25 HCO3 =10 - HCO3 - HCO3 CL- =115 + CL- CL- =105 + A- CL- =105 HYPERCHLOREMIC NORMAL HIGH AG
- 33. THE SERUM ANION GAP AG = Na+ - (HCO3- + CL-), NL = 10 Âą 2 AG âĨ 27 INDICATE ORGANIC MET. AC. AG + HCO3 âĨ 42 INDICATE MET. AL. AG DECREASED WITH PARAPROTEIN AG DECREASED WITH LOW ALBUMIN
- 34. Increased anion gap metabolic acidosis ïŽ ketones, lactate, sulfates, or metabolites of methanol, ethylene glycol, and salicylate hyperalbuminemia and uremia (increased anions) hypocalcemia or hypomagnesemia (decreased cations)
- 35. The effect of low albumin can be accounted for by adjusting the normal range for the anion gap 2.5 mEq/L for every 1 g/dL fall in albumin.
- 36. Decreased anion gap hypoalbuminemia hypercalcemia hypermagnesemia Lithium intoxication hypergammaglobulinemia bromide or iodide intoxication
- 37. ACID-BASE DISORDERS EXAMPLE OF A SIMPLE DISORDER pH (7.55) = C * [HCO3-] (18 mmol/L) PCO2 (21 mm Hg) ïŽ Step 1: pH , indicates alkalemia (Met. or Resp) ïŽ Step 2: HCO3- , indicates Resp. Alkalosis ïŽ Step 3: PCO2 , confirms Resp. Alkalosis
- 38. The delta gap The difference between the patient's anion gap and the normal anion gap is termed the delta gap considered an HCO3 â equivalent, because for every unit Rise in the anion gap, the HCO3 â should lower by 1 The delta gap is added to the measured HCO3 â , the result should be in the normal range for HCO3 â; elevation indicates the additional presence of a metabolic alkalosis
- 39. ACID-BASE DISORDERS EXAMPLE OF A MIXED DISORDER pH (7.55) = C * [HCO3-] (30 mmol/L) PCO2 (35 mm Hg) ïŽ Step 1: Alkalemia ïŽ Step 2: HCO3- , indicates Met. alkalosis ïŽ Step 3: PCO2 , indicates Resp. alkalosis ïŽ Step 4: â HCO3- (25%) > â PCO2 (12.5%) ïŽ Step 5: The major disorder is metabolic alkalosis
- 40. MIXED ACID-BASE DISORDERS DOUBLE ïŽ Metabolic and respiratory acidosis (serious) ïŽ Metabolic and respiratory alkalosis (serious) ïŽ Metabolic acidosis & respiratory alkalosis ïŽ Metabolic alkalosis & respiratory acidosis TRIPLE ïŽ Metabolic acidosis & alkalosis + resp. disorder
- 41. MIXED ACID-BASE DISORDERS EXAMPLE OF DOUBLE DISORDER Health Emphysema + Diarrhea pH 7.40 7.32 7.10 PCO2 40 80 80 HCO3 24 40 24
- 42. MIXED ACID-BASE DISORDERS EXAMPLE OF DOUBLE DISORDER Health Emphysema + Diuretic pH 7.40 7.32 7.40 PCO2 40 80 80 HCO3 24 40 48
- 43. MIXED ACID-BASE DISORDERS EXAMPLE OF TRIPLE DISORDER Health NG + Sepsis + Endotox. pH 7.40 7.49 7.14 7.44 PCO2 40 44 24 12 HCO3 24 32 8 8 AG 9 11 33 35 â AG 0 2 24 26
- 44. Masked disorder A vomiting, ill-appearing diabetic patient has laboratory results showing: ïŽ Na, 137; K, 3.8; Cl, 90; HCO3 â, 22; ïŽ pH, 7.40; Pco2, 41; Po2, 85 anion gap = 137 â (90 + 22) = 25 (normal :10) Respiratory compensation is evaluated by Winter's formula Predicted Pco2 = 1.5 (22) + 8 Âą 2 = 41 Âą 2 delta gap = 15 + 22 = 37
- 46. Normal ABG? A diabetic patient presented with gastroentritis found to have: ïŽ pH: 7.4 ïŽ HCO3: 24 ïŽ PCO2: 40 ïŽ Na: 144, K: 4, CL: 95, TCO2: 24, RBS: 520, ïŽ Positive test for ketons What is the acid-base status of this patient?
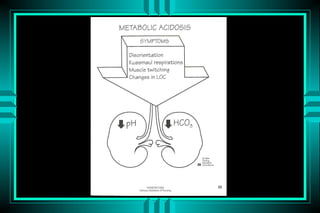
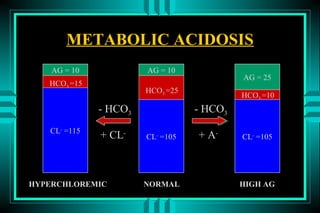
- 48. METABOLIC ACIDOSIS P R IM A R Y : D E C R E A S D H C O 3 RESPO NSE: D EC RESED PC O 2 C AU SES NO RM A L A G H IG H A G 1 0 m E q /L > 1 5 m E q /L G I HC O 3 LO SS K E T O A C ID O S ID RENA L HC O 3 LO SS L A C T IC A C ID O S IS H Y P O A L D O S T E R O N IS M R E N A L F A IL U R E TPN IN T O X IC A T IO N
- 50. METABOLIC ACIDOSIS HIGH ANION GAP Ketoacidosis (DM1, ETOH, Starvation) Lactic acidosis (A, B, D) Intoxication ïŽ Ethylene glycol ïŽ Methanol ïŽ Salicylic acid Advanced renal failure
- 52. METABOLIC ACIDOSIS INTOXICATION: HIGH OSMOLAR GAP ïŽ ETHYLENE GLYCOL ïŽ METHANOL SALICYLATE ïŽ RESPIRATORY ALKALOSIS ïŽ METABOLIC ACIDOSIS ïŽ MIXED
- 53. METABOLIC ACIDOSIS KETOACIDOSIS Diabetes 1 (Insulin lack) leads to fatty acids oxidation and production of acetoacetate (2) and B-OH-butyrate (5), which is buffered by HCO 3-, causing high AG ETOH (altered cell metabolism) Starvation (use of fatty acids), usually mild
- 54. METABOLIC ACIDOSIS LACTIC ACIDOSIS (Dx by exclusion) Type A: O2 delivery to cells is inadequate ïŽ Shock, mesenteric vascular events, and pulmonary edema) Type B: Cells cannot use O2 ïŽ Hepatic failure, sepsis, acute pancreatitis Anaerobic glycolysis of glucose to pyruvate and then lactate (buffered by HCO3-)
- 55. METABOLIC ACIDOSIS RENAL FAILURE: Unable to excrete the daily acid load Bone buffers keep HCO3-> 15 in CRF In ARF HCO3- falls by 0.5 mmol/L/day Retention of sulfate, phosphate, and organic anions causes the increase in AG
- 56. METABOLIC ACIDOSIS NORMAL ANION GAP ïŽ GI HCO3- LOSS (Diarrhea, fistula) ïŽ RENAL HCO3- LOSS ï RTA (Proximal, Distal, Hyperkalemic) ï Acetazolamide, hypoaldostironism ïŽ Miscellaneous ï NH4Cl ingestion, Sulfur ingestion ï Pronounced dilution
- 58. METABOLIC ACIDOSIS GI. BICARBONATE LOSS NORMAL AG, HYPERCHLOREMIC CAUSES ïŽ DIARRHEA ïŽ EXTERNAL FISTULA ïŽ URETEROSIGMOIDOSTOMY OR ILEAL LOOP CONDUIT
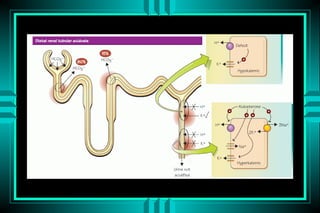
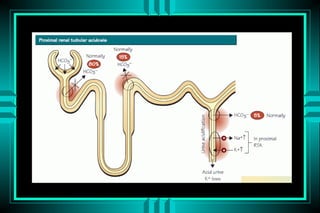
- 59. METABOLIC ACIDOSIS RENAL BICARBONATE LOSS TYPE I RTA (DISTAL, CLASSICAL) ïŽ PROTON SECRETION DEFECT TYPE II RTA (PROXIMAL, FANCONOI) ïŽ BICARBONATE REABSORPTION DEFECT TYPE IV RTA (HYPERKALEMIC) ïŽ HYPORENINEMIC HYPOALDOSTERONISM
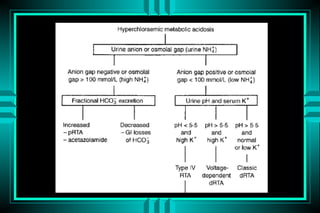
- 64. METABOLIC ACIDOSIS URINARY ANION GAP UAG = (Na+ + K+) - Cl- UAG is an estimate of urinary ammonium ïŽ Elevated in GI HCO3- loss ïŽ Low in distal RTA UAG: NEGATIVE -20 mEq/L IN GI LOSS UAG: POSITIVE + 23 mEq/L IN RTA
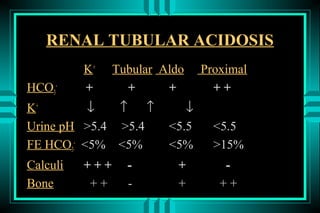
- 65. RENAL TUBULAR ACIDOSIS K+ Tubular Aldo Proximal HCO3- + + + ++ K+ â â â â Urine pH >5.4 >5.4 <5.5 <5.5 FE HCO3- <5% <5% <5% >15% Calculi +++ - + - Bone ++ - + ++
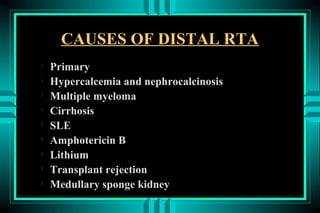
- 66. CAUSES OF DISTAL RTA ïŽ Primary ïŽ Hypercalcemia and nephrocalcinosis ïŽ Multiple myeloma ïŽ Cirrhosis ïŽ SLE ïŽ Amphotericin B ïŽ Lithium ïŽ Transplant rejection ïŽ Medullary sponge kidney
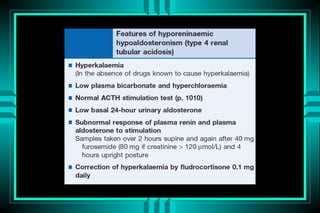
- 67. CAUSES OF HYPERKALEMIC RTA ïŽ Hypoaldosteronism ïŽ Obstructive nephropathy ïŽ Sickle cell nephropathy ïŽ SLE ïŽ Cyclosporine A nephropathy
- 68. CAUSES OF PROXIMAL RTA ïŽ Primary ïŽ Cystinosis ïŽ Wilsonâs disease ïŽ Lead toxicity ïŽ Multiple myeloma ïŽ Nephrotic syndrome ïŽ Early transplant rejection ïŽ Medullary cystic disease ïŽ Outdated tetracycline
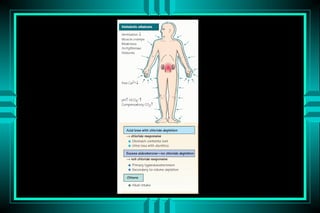
- 69. METABOLIC ALKALOSIS P r im a r y : IN C R E A S E D H C O 3 R E S P O N S E : IN C R E A S E D P C O 2 A p p r o p r ia te r e s p o n s e ? P C O 2 = 0 .7 H C O 3 U R IN A R Y C H L O R ID E < 2 0 m E q /L > 2 0 m E q /L N o rm a l E C V D e c re a s e d E C V N o rm a l E C V D e c re a s e d E C V A lk a li lo a d G I lo s s E xcess B a r tte r 's D iu r e tic s M in e r a lo c o r tic o id s H y p o k a le m ia
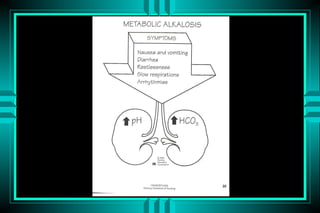
- 75. Effects of Metabolic Alkalosis Decreased serum potassium Decreased serum ionized calcium Dysrhythmias Hypoventilation / hypoxemia Increased bronchial tone / atelectasis Left shift of the Oxygen curve
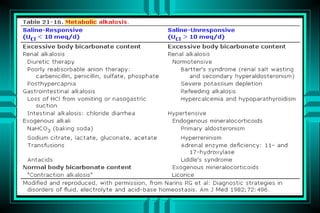
- 76. METABOLIC ALKALOSIS Volume - depleted type: ïŽ Gastric acid loss ï Vomiting ï NGT suction ïŽ Renal chloride loss ï Diuretics ï Hypercapnia correction
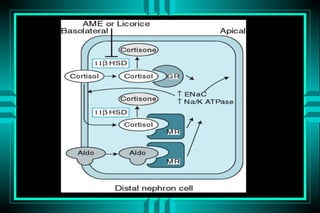
- 77. METABOLIC ALKALOSIS Volume - repleted type: ïŽMineralocorticoid excess ï Hyperaldosteronism ï Bartterâs syndrome ï Cushingâs syndrome ï Licorice excess ïŽProfound potassium depletion
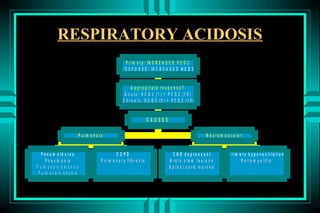
- 79. RESPIRATORY ACIDOSIS P r i m a r y : IN C R E A S E D P C O 2 R E S P O N S E : IN C R E A S E D H C O 3 A p p ro p ria te re s p o n s e ? A c u te : H C O 3 (1 ) = P C O 2 (1 0 ) C h ro n ic : H C O 3 (3 ) = P C O 2 (1 0 ) CAUSES P u lm o n a ry N e u ro m u s c u la r P n e u m o th o ra x COPD C N S d e p re s s a n t P rim a ry h y p o v e n tila tio n P n e u m o n ia P u lm o n a ry fib ro s is B ra in s te m le s io n s P o lio m y e litis P u lm o n a r y e m b o lu s S p in a l c o rd le s io n s P u lm o n a r y e d e m a
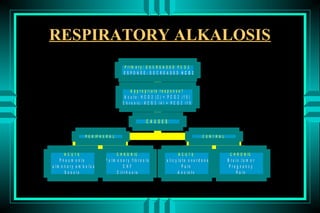
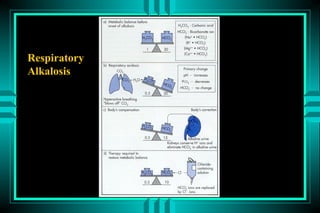
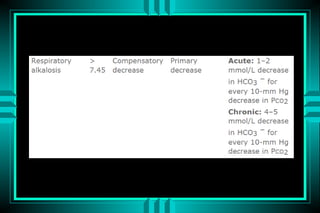
- 81. RESPIRATORY ALKALOSIS P rim a ry : D E C R E A S E D P C O 2 RESPONSE: DECREASED HCO3 A p p ro p ria te re s p o n s e ? A c u te : H C O 3 (2 ) = P C O 2 (1 0 ) C h ro n ic : H C O 3 (4 ) = P C O 2 (1 0 ) CAUSES P E R IP H E R A L CENTRAL ACUTE C H R O N IC ACUTE C H R O N IC P n e u m o n ia P u lm o n a ry fib ro s is S a lic y la te o v e rd o s e B ra in tu m o r P u lm o n a ry e m b o lu s CHF P a in P re g n a n c y S e p s is C irrh o s is A n x ie ty P a in
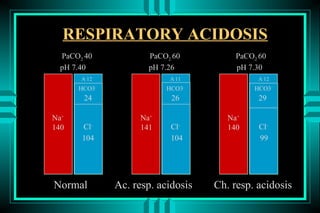
- 83. RESPIRATORY ACIDOSIS PaCO2 40 PaCO2 60 PaCO2 60 pH 7.40 pH 7.26 pH 7.30 A-12 A-11 A-12 HCO3- HCO3- HCO3- 24 26 29 Na+ Na+ Na+ 140 Cl- 141 Cl- 140 Cl- 104 104 99 Normal Ac. resp. acidosis Ch. resp. acidosis
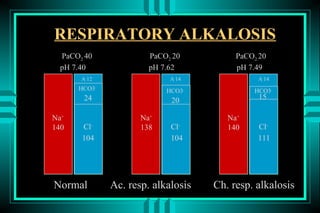
- 84. RESPIRATORY ALKALOSIS PaCO2 40 PaCO2 20 PaCO2 20 pH 7.40 pH 7.62 pH 7.49 A-12 A-14 A-14 HCO3- HCO3- HCO3- 24 20 15 Na+ Na+ Na+ 140 Cl- 138 Cl- 140 Cl- 104 104 111 Normal Ac. resp. alkalosis Ch. resp. alkalosis
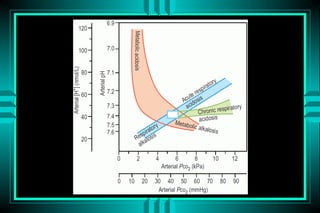
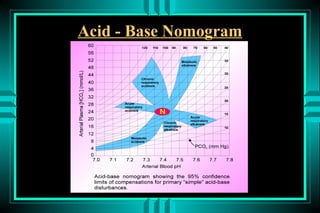
- 85. Acid - Base Nomogram
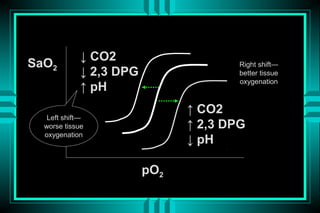
- 86. â CO2 SaO2 Right shiftâ â 2,3 DPG better tissue oxygenation â pH â CO2 Left shiftâ worse tissue â 2,3 DPG oxygenation â pH pO2
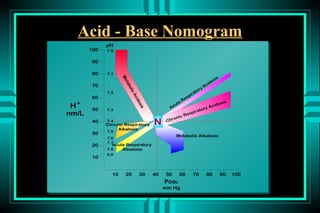
- 87. Acid - Base Nomogram









![ACID-BASE DISORDERS
FORMULAS:
HENDERSON-HASSELBALCH
ïŽ pH = pK + log ([HCO3-]/[0.03* PCO2])
ïŽ
pH = 6.10 + log (24/0.03*40) = 7.40
MODIFIED HENDERSON
ïŽ [H+] = 24* PCO2/[HCO3-]
ïŽ
[H+] = 24* (40/24) = 40 neq/L (pH=7.4)](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-17-320.jpg)













![ACID-BASE DISORDERS
EXAMPLE OF A SIMPLE DISORDER
pH (7.55) = C * [HCO3-] (18 mmol/L)
PCO2 (21 mm Hg)
ïŽ
Step 1: pH , indicates alkalemia (Met. or Resp)
ïŽ Step 2: HCO3- , indicates Resp. Alkalosis
ïŽ Step 3: PCO2 , confirms Resp. Alkalosis](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-37-320.jpg)

![ACID-BASE DISORDERS
EXAMPLE OF A MIXED DISORDER
pH (7.55) = C * [HCO3-] (30 mmol/L)
PCO2 (35 mm Hg)
ïŽ
Step 1: Alkalemia
ïŽ Step 2: HCO3- , indicates Met. alkalosis
ïŽ Step 3: PCO2 , indicates Resp. alkalosis
ïŽ Step 4: â HCO3- (25%) > â PCO2 (12.5%)
ïŽ
Step 5: The major disorder is metabolic alkalosis](https://image.slidesharecdn.com/acid-base-2013-130203032247-phpapp02/85/Acid-base-disorders-39-320.jpg)