Acid Base Equilibrium
- 2. Acid base theoriesArrhenius TheoryBrÃķnsted â Lowry TheoryLewis acid â base Theory
- 3. Acid Base behaviourIn this unit when ever we discuss acids and bases we study the behaviour of compounds when dissolved in waterThe solvent is all the time Water
- 4. Arrhenius TheoryAcid- any substance that increases the hydronium (H3O+) ion concentration in neutral water. (or H+ ions for simplicity)Base - any substance that increases the hydroxide (OH-) ion concentration in neutral water.The following pages shows some examples of acids and bases
- 5. Strong Acids = Ka is very largePerchloric acid HClO4Hydroiodic acid HIHydrobromic acid HBrSulfuric acid H2SO4Hydrochloric acid HClNitric acid HNO3Memorise these examples
- 6. Weak Acid = Ka is very small at 25oCSulfurous acid H2SO3 Ka = 1.2 x 10 -2Hydrogen sulphate ion HSO4- Ka = 1.0 x 10-2Phosphoric acid H3PO4 Ka = 7.1 x 10-4Citric acid H3C6H5O7 Ka = 7.1 x 10-4Nitrous acid HNO2 Ka = 7.1 x 10-4Hydrofluoric acid HF Ka = 6.8 x 10-4Formic Acid HCO2H Ka = 1.8 x 10-4
- 7. Weak Acids continued at 25oCAcetic acid HC2H3O3 Ka = 1.8 x 10-5Carbonic acid H2CO3 Ka = 4.5 x 10-7Hydrogen cyanide HCN Ka = 6.2 x 10-10Ammonium ion NH4+ Ka = 5.7 x 10-10Bicarbonate ion HCO3- Ka = 4.7 x 10-11Water H2O Ka = 1.8 x 10-16
- 8. Arrhenius BasesSodium hydroxide NaOHAmmonium hydroxide NH4OHCalcium hydroxide Ca(OH)2 (Lime water)
- 9. Kw and pHWater is weakly dissociated and the equilibrium can be shown like thisH2O + H2O = H3O+ + OHH2O = H+ + OH-Kw = [H+][OH-]Kw = 10-14 at 250C for water H2O = H++ OH--x +x +xKw = x2x = = 10-7
- 10. pHpH = -log[H+]pOH = -log[OH-]For water pH = -log[10-7] = - [-7log 1o] = 7Similarly pOH = 7pH + pOH = 14pOH = 14-pHpH = 14 - pOH
- 11. BrÃķnsted Lowry TheoryAn acid is a proton donorA base is a proto acceptorAn amphoteric substance is one that can act both as an acid and a base.Amphoteric substances are proton acceptors or donors eg. H2O, HCO3-, etc.
- 12. BrÃķnsted acids bases examplesNH3 + H2O ïŪ     NH4+   +    OH- base    acid          conjugate  conjugate                                 acid            base HCl + H2O ïŪ     H3O+   +       Cl-acid   base             conjugate      conjugate                                       acid               base
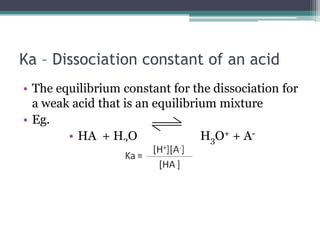
- 13. Ka â Dissociation constant of an acidThe equilibrium constant for the dissociation for a weak acid that is an equilibrium mixtureEg.HA + H2O H3O+ + A-
- 14. Kb = Dissociation constant of a baseNH3(aq) + H2O NH4+(aq) + OH-(aq)CO32-(aq) + H2O HCO3-(aq) + OH-(aq)B (aq) + H2O BH+(aq) + OH- (aq)
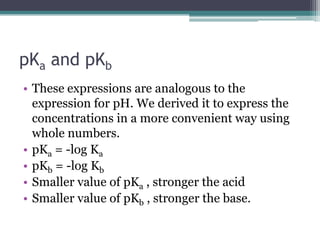
- 15. pKa and pKbThese expressions are analogous to the expression for pH. We derived it to express the concentrations in a more convenient way using whole numbers.pKa = -log KapKb = -log KbSmaller value of pKa , stronger the acidSmaller value of pKb , stronger the base.
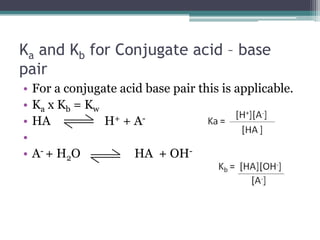
- 16. Ka and Kb for Conjugate acid â base pairFor a conjugate acid base pair this is applicable.Ka x Kb = KwHA H+ + A-A- + H2O HA + OH-
- 17. Conjugate acid base pairsKa x Kb
- 18. Relationships between Ka, Kb,Kw.
- 19. Calculations involving KaCalculate the value of Ka for a specific acid from the [H+] or pH of a solution for which we also know the initial concentration of the acidCalculate the equilibrium concentrations of H+ and A- from the initial concentration of a specific weak acid and its Ka value
- 20. Calculations involving KbCalculate the value of Ka for a specific acid from the [OH-] or pOH of a solution for which we also know the initial concentration of the base or from pH as pOH = 14 - pHCalculate the equilibrium concentrations of [H+ ]or [OH-]from the initial concentration of a specific weak base and its Kb value (Or what is almost the same is calculating the pH or pOH from the values of Kb and initial concentration of base)
- 21. Hydrolysis of IonsThere are many ionic saltâs that make the aqueous solution acidic or basic.The reaction of ionic salts with water is called âHydrolysis of saltsâHydrolysis can be explained using BrÃķnsted Lowry theory.If the conjugate acid or base is strong then there will be hydrolysis.Weak acids and bases produce strong conjugate bases and acids
- 22. Illustration of HydrolysisA- is a strong conjugate base A-(aq)+ H2O = HA(aq) + OH-The dissociation constant for the hydrolysis in this case is Kb as one of the products is a base.
- 23. Hydrolysis of strong conjugate acidNH4+ + H2O = H3O+ + NH3NH4+ is a strong conjugate acid therefore undergoes hydrolysis and the solution becomes more acidic or pH is lowered.Other positive metal ions that can undergo hydrolysis is Fe3+, Al3+ etc.âĶâĶâĶâĶâĶâĶ
- 24. Titration of weak acid Vs baseAcetic acid Vs NaOH
- 26. Polyprotic acidFor any diprotic acid the value of Ka1 > Ka2It is easier to pull a H+ from a neutral molecule than an ionKa1 is usually 104 to 105 times larger than Ka2. Therefore the contribution of acidity due to the second or third proton is negligible and can be ignored.Â
- 27. Calculating Ka from pH #1Formic acid HCHO2 is a monoprotic acid. In a 0.100 M solution of formic acid, the pH is 2.38 at 25oC. Calculate Ka for formic acid at this temperature.Ans: Ka = 1.8x10-4
- 28. #1Determine the [H+] ion concentration from pHMake Ice table sand substitute the concentration of H+ in the table and complete it to get the equilibrium concentration of HA, H+ and A-Use the values to determine the Ka for the acid.
- 29. Calculating [H+], pH from Ka for weak acid #2The concentration of vinegar HC2H3O2 was found to be 0.75 M acetic acid, Calculate the value for the [H+] ion concentration and pH for this acid solution if Ka for acetic acid is 1.8 x 10-5
- 30. #2Create an ice table initial concentration is 0.75 M for acetic acid. Change in concentration is âx,x and x respectively for HA, H+ and A-Since Ka is very small 0.75 âx can be taken as 0.75 and solve for x.X is the concentration of H+ use this value to calculate pH








![Kw and pHWater is weakly dissociated and the equilibrium can be shown like thisH2O + H2O = H3O+ + OHH2O = H+ + OH-Kw = [H+][OH-]Kw = 10-14 at 250C for water H2O = H++ OH--x +x +xKw = x2x = = 10-7](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-9-320.jpg)
![pHpH = -log[H+]pOH = -log[OH-]For water pH = -log[10-7] = - [-7log 1o] = 7Similarly pOH = 7pH + pOH = 14pOH = 14-pHpH = 14 - pOH](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-10-320.jpg)





![Calculations involving KaCalculate the value of Ka for a specific acid from the [H+] or pH of a solution for which we also know the initial concentration of the acidCalculate the equilibrium concentrations of H+ and A- from the initial concentration of a specific weak acid and its Ka value](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-19-320.jpg)
![Calculations involving KbCalculate the value of Ka for a specific acid from the [OH-] or pOH of a solution for which we also know the initial concentration of the base or from pH as pOH = 14 - pHCalculate the equilibrium concentrations of [H+ ]or [OH-]from the initial concentration of a specific weak base and its Kb value (Or what is almost the same is calculating the pH or pOH from the values of Kb and initial concentration of base)](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-20-320.jpg)







![#1Determine the [H+] ion concentration from pHMake Ice table sand substitute the concentration of H+ in the table and complete it to get the equilibrium concentration of HA, H+ and A-Use the values to determine the Ka for the acid.](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-28-320.jpg)
![Calculating [H+], pH from Ka for weak acid #2The concentration of vinegar HC2H3O2 was found to be 0.75 M acetic acid, Calculate the value for the [H+] ion concentration and pH for this acid solution if Ka for acetic acid is 1.8 x 10-5](https://image.slidesharecdn.com/acidbaseequilibrium2-110505210538-phpapp02/85/Acid-Base-Equilibrium-29-320.jpg)
