Air and solid waste sampling
- 1. SAMPLING PROCEDURE FOR AIR AND SOLID WASTE SUBMITTED TO: Dr. MEENA THAKUR SUBMITTED BY: YASHPAL (F-14-21-M)
- 2. SAMPLING: ï‚—Sampling is the process of collection of represented sample from the whole of the population. SAMPLE: ï‚—Part of population consisting of one or more sampling units, selected and examined as a representive of whole.
- 4. SAMPLING OF AIR POLLUTANTS First step in analysis of air pollutant. May be present either alone or in combinations. Due to the difference in physical states and the variability in ambient concentrations, common devices cannot be applied for all kinds of pollutant.
- 5. HOW TO HAVE A GOOD SAMPLE Size of sample: 10 m3 Sampling rate : 0.003- 3.0 m3/min. Sampling time : 3 hour
- 6. COLLECTION OF GASEOUS AIR POLLUTANTS Four methods: 1.Grab sampling 2.Absorption in liquid 3.Absorption on solids 4.Freeze out sampling
- 7. GRAB SAMPLING ï‚—The sample is collected by filling an evacuated flask or an inflatable bag. ï‚—Plastic bags , glass and stainless containers are widely used for grab sampling. ï‚—The containers are evacuated and filled by allowing air to enter. A container may be filled with water and then used as a collector simply by draining away the water which is replaced by a air. ï‚—Heating tapes and nichrome wires are used to prevent condensation during sampling.
- 8. ABSORPTION IN LIQUID Most common method of collecting the samples. Separates the desired pollutants from air either through direct solubility in absorbing medium or by chemical reaction. Collectors used: to provide high degree of gas liquid contact.  Fritted absorber  Impingers
- 9. ABSORPTION ON SOLID Based on tendency of gasses to be absorbed on surfaces of solid material. Sample air is passed through a packed column containing a finely divided solid adsorbent on whose surface the pollutants are retained and concentrated. Activated charcoal and silica gel are most commonly used solid adsorbents.
- 10. FREEZE OUT SAMPLING Contains a series of cold traps. Maintained at low temp. Used to draw the air sample whereby the pollutants are condensed. Traps are brought to lab and analyzed by means of gas chromatography, IR & UV spectrophotometry, mass spectrometry or by wet chemical means.
- 11. SAMPLING OF PARTICULATE MATTER Five methods: 1.Filteration 2.Sedimentation 3.Electrostatic precipitator 4.Thermal precipitator 5.Impingers.
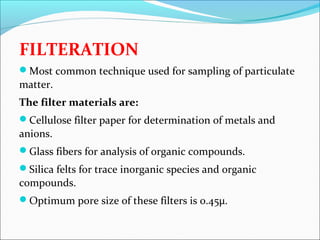
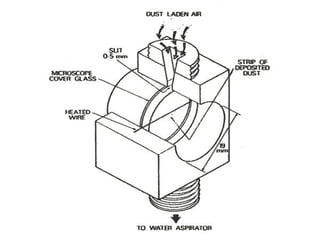
- 12. FILTERATION Most common technique used for sampling of particulate matter. The filter materials are: Cellulose filter paper for determination of metals and anions. Glass fibers for analysis of organic compounds. Silica felts for trace inorganic species and organic compounds. Optimum pore size of these filters is 0.45µ.
- 13. SEDIMENTATION Suitable for comparatively larger particles having size more than of 10µ. Apparatus used is simple glass jar fitted with a funnel. A liquid is added to the collector so that solids are prevented from being blown out of air. Sample is deposited over a period of 1 month and the material is dried and weighed.
- 14. ELECTROSTATIC PRECIPITATOR Very efficient for collection of small particles. Particles are collected on circular plate. On entering the sampler, particles pick up charge as they pass through an electric discharge between two electrodes maintained at a potential differences of up to 3000 volts. Small charged particle in gas stream loose their charge in contact with electrode(oppositely charged) and get accumulated on the electrode.
- 16. THERMAL PRECIPITATORTHERMAL PRECIPITATOR Based on a principle of thermal force which deflects the particles from the zone of lower temp. to the zone of higher temp. Used for the collection of particles of 0.001µ.
- 18. IMPINGERSIMPINGERS It collect particles from a relatively high velocity air stream directed at a surface. Device is a dry impinger when collecting surface is dry and wet impinger when surface is wet.
- 19. SAMPLING OF GASES AND VAPOURSSAMPLING OF GASES AND VAPOURS Four methods: 1.Bags and containers. 2.Absorption. 3.Adsorption. 4.Cold trapping.
- 20. COLD TRAPPINGCOLD TRAPPING All particles from an air stream may be removed by cold trapping i.e. freezing & liquefying gases and vapours in collectors maintained at a low temperature. Fractionation is achieved by using collector maintained at progressively low temperature. The first collector is maintained in an ice bath & the last collector in a liquid nitrogen bath (-196C).
- 21. ABSORPTIONABSORPTION Method of collection of gaseous pollutant is absorption in a solvent, such as by bubbling the gas through a liquid. Pure water is adequate for collecting some gaseous pollutants such as HF. Alkaline solution for absorbing acidic gases, acidic solution for absorbing alkaline gases and oils for collecting hydrocarbons.
- 22. ADSORPTIONADSORPTION Charcoal is efficient adsorber for gases and vapours in air. Air is pumped through the tube at a rate of 1L/min. After adsorption charcoal is washed with suitable solvent such as CS₂. Beside charcoal silica gels, aluminium oxides are also used as adsorber.
- 23. SAMPLE PRESERVATIONSAMPLE PRESERVATION AND STORAGEAND STORAGE PURPOSE : To minimize physical, chemical and biological changes Three approaches: 1. Refrigeration 2. Use of proper sample container 3. Addition of preserving chemicals.
- 24. Refrigeration: to slow down loss processes Container choice (material type and headspace) is critical to reduce – Volatilization – Adsorption – Absorption – Diffusion – Photodegradation Addition of preservatives is critical to reduce losses due to chemical reactions and bacterial degradation.
- 26. PROCEDURE: 1. Upload a truckload of wastes in a controlled area away from other operations. 2. Quarter the wasteload. 3. Select one of the quarters and quarter the quarter. 4. Select one of the quartered quarters and separate all of the individual components of the waste into preselected components.
- 27. 5. Place the separated components in a container of known volume and tare mass and measure the volume and mass of each component. 6. Determine the percentage distribution of each component by mass and the as-discarded density.  100-200 kg of waste should be sorted to obtain a representative sample.
- 28. ï‚—Prepare 100-200 kg of Waste from waste pit / collection vehicle
- 29. Mix and flatten the waste Divide into 4 blocks
- 30. Remove 2 blocks of Waste diagonally opposite ends Another diagonal opposite ends waste are remained
- 31. Remained waste after 3-4 reduction Prepare 2 buckets to accommodate remained waste
- 32. If the purpose of the waste analysis is for plan for waste incineration system, then waste will be dried to make it easy to proceed next steps. But, in case of improvement of collection / final disposal, waste component study will be made by wet base (without drying).
- 33. THANK YOU