Aizant Corporate Ppt
- 1. Aizant Drug Research Solutions Integrated Drug Development Solutions 7/3/2012 Confidential 1
- 2. Flow of presentation • Introduction • Business verticals – CDO Capabilities – CRO Capabilities • Support functions • Advantage • Contacts 7/3/2012 Confidential 2
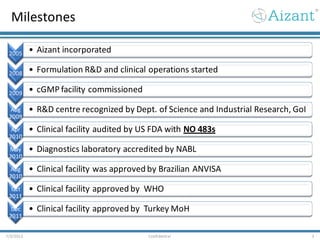
- 3. Milestones 7/3/2012 3Confidential 2005 • Aizant incorporated 2008 • Formulation R&D and clinical operations started 2009 • cGMP facility commissioned Aug 2009 • R&D centre recognized by Dept. of Science and Industrial Research, GoI Apr 2010 • Clinical facility audited by US FDA with NO 483s May 2010 • Diagnostics laboratory accredited by NABL Aug 2010 • Clinical facility was approved by Brazilian ANVISA Oct 2011 • Clinical facility approved by WHO Dec 2011 • Clinical facility approved by Turkey MoH
- 4. Vision & Mission 7/3/2012 Confidential 4 • To be a global leader for science based integrated drug developmentsolutionsVISION • Pursuit of excellence through science and technology • Agile team with open communication and honoring deliverables • Environmentally and socially responsible research MISSION • Innovation • People • Learning • Quality VALUES
- 5. Quality • Quality is by design and not an after thought • QbD is fundamental to our operations – Targetthe product profile (TPP) – Determine the critical quality attributes (CQAs) – Link input material attributes and process parameters to CQAs and perform risk assessment – Develop a design space – Designand implement a control strategy – Manageproduct lifecycle, including continual improvement 7/3/2012 Confidential 5
- 6. Flow of presentation • Introduction • Business verticals – CDO Capabilities – CRO Capabilities • Support functions • Advantage • Contacts 7/3/2012 Confidential 6
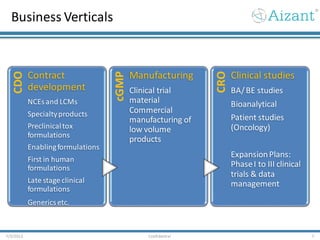
- 7. Business Verticals 7/3/2012 Confidential 7 CDO Contract development NCEs and LCMs Specialtyproducts Preclinicaltox formulations Enablingformulations First in human formulations Late stage clinical formulations Generics etc. cGMP Manufacturing Clinical trial material Commercial manufacturing of low volume products CRO Clinical studies BA/BE studies Bioanalytical Patient studies (Oncology) ExpansionPlans: PhaseI to III clinical trials & data management
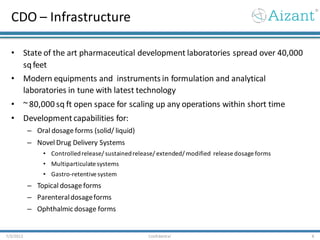
- 8. CDO – Infrastructure • State of the art pharmaceutical development laboratories spread over 40,000 sqfeet • Modern equipments and instruments in formulation and analytical laboratories in tune with latest technology • ~ 80,000 sq ft open space for scaling up any operations within short time • Developmentcapabilities for: – Oral dosage forms (solid/ liquid) – Novel Drug Delivery Systems • Controlledrelease/ sustainedrelease/ extended/ modified release dosage forms • Multiparticulate systems • Gastro-retentive system – Topical dosage forms – Parenteraldosageforms – Ophthalmicdosage forms 7/3/2012 Confidential 8
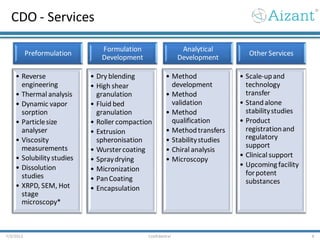
- 9. CDO - Services 7/3/2012 Confidential 9 Preformulation • Reverse engineering • Thermal analysis • Dynamic vapor sorption • Particlesize analyser • Viscosity measurements • Solubility studies • Dissolution studies • XRPD, SEM, Hot stage microscopy* Formulation Development • Dry blending • High shear granulation • Fluid bed granulation • Roller compaction • Extrusion spheronisation • Wurstercoating • Spraydrying • Micronization • PanCoating • Encapsulation Analytical Development • Method development • Method validation • Method qualification • Methodtransfers • Stabilitystudies • Chiral analysis • Microscopy Other Services • Scale-upand technology transfer • Standalone stabilitystudies • Product registrationand regulatory support • Clinical support • Upcomingfacility forpotent substances
- 10. cGMP – Infrastructure • About 10,000 sq. feet cGMP area for scale up and clinical batch manufacturing • Designed to meet US FDA/ MHRA standards • DedicatedAHUs • Flexibility of manufacturing batches from 0.05kg to 80kgs • Power back to ensure smooth operations • EnsuresOSHA compliance and other industry legislations • Process train for solid orals: – Up to 15 kg – Up to 100 kg 7/3/2012 Confidential 10
- 11. cGMP - Services 7/3/2012 Confidential 11 Scale up • Scaleup of formulation development products • Manufacturing batchesfor regulatory submissions • Commercial manufactureof low volume products • Allkinds of packaging Clinical trial material • Investigational drug product • Placebo • Encapsulationof tablets, multiparticulate, capsulesand other solid dosage forms • Comparator manufacturing • Clinical packaging, blinding, randomization Analytics • Testingand release of finishedgoods • Cleaning validation/ process validation • Microbiology • Standalone stabilitytesting Other Services • Regulatory support
- 13. Differentiating technologies offered • Nanotechnology – Liposomes – Nanoparticles – Dendrimers – Nanofibers – Polymeric micelles • Hot Melt Extrusion (HME) – Bioavailabilityenhancement – Stability enhancement – Controlled release formulations – Product life cycle extension – Tastemasking and Pediatric dosageform – Costreduction 7/3/2012 Confidential 13
- 14. Differentiating technologies offered • Potentsubstances • Parenteral development – Liposomes – Lyophilized products • Spray drying • Self Enabling Drug Delivery Systems(SEDDS) 7/3/2012 Confidential 14
- 15. CRO – Infrastructure • 80 bed (2 clinics) facility spread over 28,000 sq feet • IP based cameras to virtually monitor projects • Dedicated registration area • Independent ethics committee • In-house NABL accredited clinical diagnostics laboratory • In-house kitchen • Bioanalytical laboratories equipped with 7 LCMS/MS including API 5500 • Offsite storage of data for disaster recovery and business continuity • Volunteer database • Male: 4000+ • Female: 600+ • Access to post menopausal women database 7/3/2012 Confidential 15
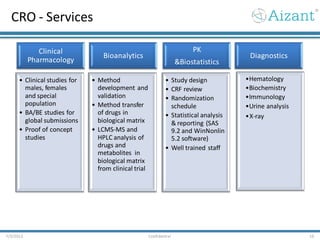
- 16. CRO - Services 7/3/2012 Confidential 16 Clinical Pharmacology • Clinical studies for males, females and special population • BA/BE studies for global submissions • Proof of concept studies Bioanalytics • Method development and validation • Method transfer of drugs in biological matrix • LCMS-MS and HPLC analysis of drugs and metabolites in biological matrix from clinical trial PK &Biostatistics • Study design • CRF review • Randomization schedule • Statistical analysis & reporting (SAS 9.2 and WinNonlin 5.2 software) • Well trained staff Diagnostics •Hematology •Biochemistry •Immunology •Urine analysis •X-ray
- 17. CRO - Regulatory approvals • US FDA audited facility with NO 483s • ANVISA inspection successfullycompleted (No major or critical observations) • NABL accreditation for clinical diagnostics • DCGI inspected and approved • WHO approved • Turkey MoH approved • Working on EMEA inspection 7/3/2012 Confidential 17
- 18. Certifications- ANVISA & FDA 7/3/2012 Confidential 18
- 19. Certifications– WHO and NABL 7/3/2012 Confidential 19
- 20. Flow of presentation • Introduction • Business verticals – CDO Capabilities – CRO Capabilities • Support functions • Advantage • Contacts 7/3/2012 Confidential 20
- 21. Project management • Cross functional project teams from formulation, analytical, quality and regulatory departments • All projects teams are monitored on MS projects by an experienced project manager • Metrics based project planning and execution • Well defined communicationsystems as decided at beginning of project • Good track record of completing projects on time 7/3/2012 Confidential 21
- 22. Informationtechnology • Data-centrewith backup and recovery facility • Offsitedata storage for disaster recovery • IP Cameras for remote virtual monitoring of projects • Biometrics for cross-check and validation • Well defined IT policy for security and access control • Metrics based reporting 7/3/2012 Confidential 22
- 23. Key Management Team & Scientists 7/3/2012 Confidential 23 S.No. Name Designation Qualification Years of experience Patents and publications Area of specialization 1 Varma Rudraraju Chairman and Managing Director MS (Pharmaceutics), University of Mississippi, USA Ph.D. (Pharmaceutics), University of Mississippi, USA 20 21 publications Preformulation to commercial manufacturing including clinical Business strategy 2 Ashok Illpakurthy DirectorProduct Development MS (Pharmaceutics), University of Mississippi, USA Ph.D. (Pharmaceutics), University of Mississippi, USA 14 7 publications Formulation development QbD, DoE Certified “Chemical Design for Six Sigma” (CDFSS) black belt 3 Narasimhan K Associate Director Analytical Development MS (Physical Chemistry), Bombay University, Mumbai MS (Analytical Chemistry), SIU, Carbondale, IL, USA Ph.D., (Analytical Chemistry), Purdue University, IN, USA 11 17 Publications; Oral presentations Analytical development and validations; Bioanalytical; Solid state chemistry Process development; QbD, DoE; Stability studies Totalteam of 300 + people between Product development,clinical developmentandGMP business verticals
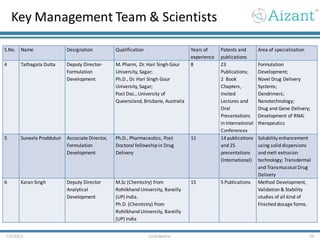
- 24. Key Management Team & Scientists 7/3/2012 Confidential 24 S.No. Name Designation Qualification Years of experience Patents and publications Area of specialization 4 Tathagata Dutta Deputy Director- Formulation Development M. Pharm, Dr. Hari Singh Gour University, Sagar; Ph.D., Dr. Hari Singh Gour University, Sagar; Post Doc., University of Queensland, Brisbane, Australia 8 23 Publications; 2 Book Chapters, Invited Lectures and Oral Presentations in International Conferences Formulation Development; Novel Drug Delivery Systems; Dendrimers; Nanotechnology; Drug and Gene Delivery; Development of RNAi therapeutics 5 Suneela Prodduturi Associate Director, Formulation Development Ph.D., Pharmaceutics, Post Doctoral fellowship in Drug Delivery 11 14 publications and 25 presentations (International) Solubility enhancement using solid dispersions and melt extrusion technology; Transdermal and Transmucosal Drug Delivery 6 Karan Singh Deputy Director Analytical Development M.Sc (Chemistry) from Rohilkhand University, Bareilly (UP) India. Ph.D. (Chemistry) from Rohilkhand University, Bareilly (UP) India 15 5 Publications Method Development, Validation & Stability studies of all kind of Finished dosage forms.
- 25. Key Management Team & Scientists 7/3/2012 Confidential 25 S.No. Name Designation Qualification Years of experience Patents and publications Area of specialization 7 Rajan Kombu Subramanian Senior Manger, Bioanalytical Department M.Pharm, Gujrat University; Ph.D., Kakatiya University; MS, Case Western Reserve University, USA 9 Patent-1; Publications- 21; Book Chapters- 1 Bioanalytical method development and validation using GC-MS and LC-MS/MS 8 Ramesh Mattupalli DirectorQuality Assurance M.Pharm, (Pharmacognosy) Banglore University 12 None Quality assurance 9 Anand Bhogu Vice President- Clinical Development MSc (Biotech), MPhil (National Chemical Laboratory, CSIR, Pune, India) 14 None Project Management BA BE studies (healthy and patient based PK studies), Discovery, Phase I, Phase II- III, Business Development
- 26. Flow of presentation • Introduction • Business verticals – CDO Capabilities – CRO Capabilities • Support functions • Advantage • Contacts 7/3/2012 Confidential 26
- 27. Experience 7/3/2012 Confidential 27 Projects Handled over 150 developmentand 200 + clinical projects since inception development projects include: Simple IR , complex IR, Simple MR, complex MR, Combination products, LCMs, NCEs Geography Rich experience of working in various regulatory environment USA, EU, LatAm, Worldwide, RoW Clients Versatileinworking with various partners Big Pharma, Specialty, Generics, Indian Generics
- 28. ExpansionPlans • Adding potent development suite (completedevelopment under isolators) for handling highly potent molecules • Adding another 40 bed clinic for handing BA/BE studies 7/3/2012 Confidential 28
- 30. Contacts 7/3/2012 Confidential 30 Aizant Drug Research Solutions Pvt. Ltd. Sy.No. 172 & 173, Apparel ParkRoad, Dulapally,Hyderabad,India– 500014 Phone:+91 40 23792190 E-mail:praveen.s@aizant.com,Website:www.aizant.com