Alcohol in organic chemistry
- 1. Alcohol ŌĆ£Their Structures, Physiochemical Properties and PreparationŌĆØ Saad Anwar Microbiologis t Topic:
- 2. Structure and Physical Properties ALCOHOL ’ü▒An organic compound containing a hydroxyl group attached to an alkyl group. ’üČa non-polar (alkane-like) chain. ’üČa polar hydroxyl group. ’ü▒Alcohols have the general formula: R-OH, where ŌĆ£RŌĆØ involves a saturated C-atom (bound to hydrogen's and/or other carbons). ’ü▒For example:
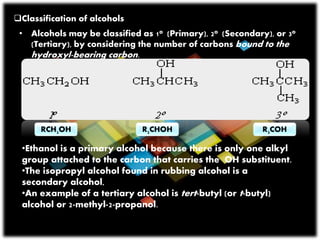
- 3. ’ü▒Classification of alcohols ŌĆó Alcohols may be classified as 1o (Primary), 2o (Secondary), or 3o (Tertiary), by considering the number of carbons bound to the hydroxyl-bearing carbon. ŌĆóEthanol is a primary alcohol because there is only one alkyl group attached to the carbon that carries the OH substituent. ŌĆóThe isopropyl alcohol found in rubbing alcohol is a secondary alcohol, ŌĆóAn example of a tertiary alcohol is tert-butyl (or t-butyl) alcohol or 2-methyl-2-propanol. RCH2OH R3COHR2CHOH
- 4. ’ü▒R-O-H has a structure similar to that of water ’ü▒Higher boiling points. Hydrogen Bond in Water Hydrogen Bond in Alcohol
- 5. ’ü▒Classification of alcohols ’ü▒Solubility ’ü▒The Lower the molecular weight of alcohols, the higher the solubility in water. ’ü▒The water-solubility of alcohols depends on the length of the alkyl chain in the alcohol. ’ü▒alcohols having chains longer than four carbons are not very water-soluble. ’ü▒3-4 carbons or lessŌĆö ARE soluble in water ’ü▒Alcohols with more than one hydroxyl group (polyhydroxyl alcohols) have higher boiling points than monohydroxyl alcohols. ’ü▒We already saw that the boiling points of alkanes increase with increasing chain length. The same is true for alcohols.
- 6. O CH3 H O CH3 H ’ü▒Alcohols having chains SHORTER than four carbons are very water-solubleŌĆ” ’ü▒ Methanol is Soluble and Miscible in waterŌĆ” Methanol
- 7. Butanol ’ü▒Alcohols having chains LONGER than Three carbons are not very water-solubleŌĆ” ’ü▒ Butanol is Soluble but not Miscible in waterŌĆ”
- 8. Formula Name Solubility in Water (g/100 g) CH3OH methanol infinitely soluble CH3CH2OH ethanol infinitely soluble CH3(CH2)2OH propanol infinitely soluble CH3(CH2)3OH butanol 9 CH3(CH2)4OH pentanol 2.7 CH3(CH2)5OH hexanol 0.6 CH3(CH2)6OH heptanol 0.18 CH3(CH2)7OH octanol 0.054 CH3(CH2)9OH decanol insoluble in water Solubilities of ALCOHOL in water
- 9. ’ü▒Approximately 600,000 students per year are assaulted by a drinking student. ’ü▒Research suggests that women are more vulnerable than men to many alcohol- induced problems. Some of these include, Organ Damage, Trauma, Legal and interpersonal difficulties. ’ü▒Alcohol affects men and women differently. Women become more impaired than men from drinking the same amount of alcohol. This is because women: ŌĆó Are generally smaller in size ŌĆó Have less body water ŌĆó Have less dehydrogenase. ŌĆó Have more estrogen ’ü▒Alcohol is a central nervous system depressant. In small amounts it can have a relaxing effect.
- 10. Preparation of Alcohols 1.From alkenes (i) By acid catalysed hydration: Alcohols can be prepared by following methods (ii) By hydroborationŌĆōoxidation: 2. By reduction of carboxylic acids and esters: 3. By Fermentation Glucose + yeast ------’āĀ alcohol + carbon dioxide
- 11. Preparation of Alcohols By Fermentation How is alcohol made? Alcohol is commercially produced using a process called fermentation. Many other alcohols can be made this way, but are more likely to be produced by synthetic routes - from natural gas, oil or coal. Fermentation? Fermentation is the process in which yeast breaks down sugar into alcohol and carbon dioxide. Yeast are tiny single-celled fungi that contain special enzymes responsible for this reaction. The word equation for this process is: Glucose + yeast --------’āĀ alcohol + carbon dioxide Carbon dioxide gas bubbles out of the fermenting solution into the air leaving a mixture of ethanol and water. It's important that no air is present or the yeast will produce ethanoic acid - the chemical found in vinegar.
- 12. Preparation of Alcohols By Fermentation In this process {NADH} donates its electrons to a derivative of pyruvate, producing ethanol. Going from pyruvate to ethanol is a two-step process. In the first step, a carboxyl group is removed from pyruvate and released in as carbon dioxide, producing a two-carbon molecule called acetaldehyde. In the second step, {NADH} passes its electrons to acetaldehyde, regenerating {NAD+} start forming ethanol.
- 13. Chemical reactions of alcohols Sure thing! Treating alcohols with HCl, HBr, or HI (which all fall under the catch-all term ŌĆ£HXŌĆØ where X is a halide) results in the formation of alkyl halides. This occurs in a two step process: first, the alcohol is protonated to give its conjugate acid. Secondly, a substitution occurs. Halogenation of alcohol