Alkynes, characteristics, naming, and synthesis
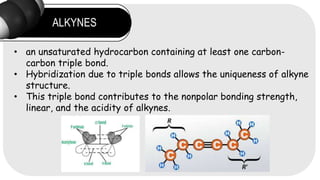
- 2. ALKYNES ? an unsaturated hydrocarbon containing at least one carbon- carbon triple bond. ? Hybridization due to triple bonds allows the uniqueness of alkyne structure. ? This triple bond contributes to the nonpolar bonding strength, linear, and the acidity of alkynes.
- 3. STRUCTURE AND PROPERTIES TERMINAL vs INTERNAL Alkynes are organic molecules with carbon-carbon triple bonds. They are unsaturated hydrocarbons with the empirical formula of CnH2n- 2. The simplest alkyne is ethyne which has the common name acetylene. Acetylene is a common name to memorize.
- 4. STRUCTURE AND PROPERTIES TERMINAL vs INTERNAL It is important to distinguish between terminal and internal alkynes because they can undergo different patterns of reactivity.
- 5. STRUCTURE AND PROPERTIES ELECTRONIC STRUCTURE The sp hybridization of carbon in a triple bond leads to a perpendicular orientation of one sigma bond and two pi bonds, making these molecules less stable. This structure affects the reactivity and acidity of alkynes, especially terminal alkynes. Due to the linear 180? bond angle of sp-hybridized carbon, a carbon ring must have at least ten members to incorporate a triple bond without excessive strain.
- 6. NOMENCLATURE OF ALKYNES Alkynes are organic molecules made of the functional group carbon-carbon triple bonds and are written in the empirical formula of CnH2n?2. They are unsaturated hydrocarbons. Like alkenes have the suffix ¨Cene, alkynes use the ending ¨Cyne; this suffix is used when there is only one alkyne in the molecule. If a molecule contains both a double and a triple bond, the carbon chain is numbered so that the first multiple bond gets a lower number.
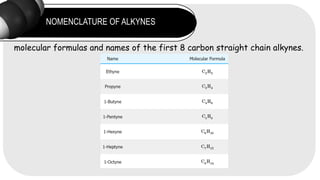
- 7. NOMENCLATURE OF ALKYNES molecular formulas and names of the first 8 carbon straight chain alkynes.
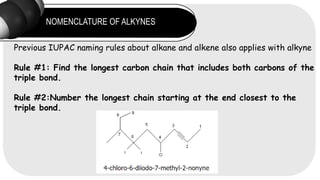
- 8. NOMENCLATURE OF ALKYNES Previous IUPAC naming rules about alkane and alkene also applies with alkyne Rule #1: Find the longest carbon chain that includes both carbons of the triple bond. Rule #2:Number the longest chain starting at the end closest to the triple bond.
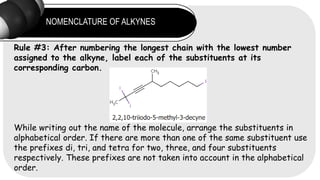
- 9. NOMENCLATURE OF ALKYNES Rule #3: After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively. These prefixes are not taken into account in the alphabetical order.
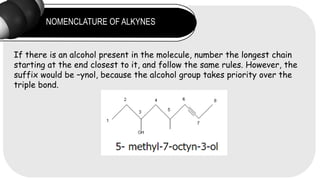
- 11. NOMENCLATURE OF ALKYNES If there is an alcohol present in the molecule, number the longest chain starting at the end closest to it, and follow the same rules. However, the suffix would be ¨Cynol, because the alcohol group takes priority over the triple bond.
- 12. NOMENCLATURE OF ALKYNES When there are two triple bonds in the molecule, find the longest carbon chain including both the triple bonds. Number the longest chain starting at the end closest to the triple bond that appears first. The suffix that would be used to name this molecule would be ¨Cdiyne.
- 13. NOMENCLATURE OF ALKYNES Rule #4: Substituents containing a triple bond are called alkynyl.
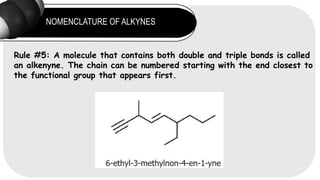
- 14. NOMENCLATURE OF ALKYNES Rule #5: A molecule that contains both double and triple bonds is called an alkenyne. The chain can be numbered starting with the end closest to the functional group that appears first.
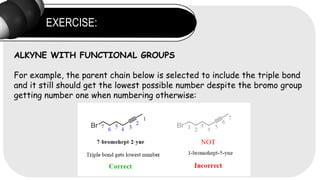
- 16. EXERCISE: ALKYNE WITH FUNCTIONAL GROUPS For example, the parent chain below is selected to include the triple bond and it still should get the lowest possible number despite the bromo group getting number one when numbering otherwise:
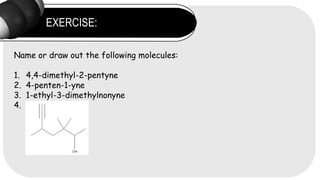
- 18. EXERCISE: Name or draw out the following molecules: 1. 4,4-dimethyl-2-pentyne 2. 4-penten-1-yne 3. 1-ethyl-3-dimethylnonyne 4.