Ames test
- 1. AMES TEST K.Krupa sagar 17M71S0105 M.Pharmacy 1st year Annamacharya college of pharmacy.
- 2. WHAT IS AMES TEST ? ŌŚ”It is a test to determine the mutagenic activity of chemicals by observing whether they cause mutations or NOT
- 3. ŌŚ” MUTATIONS: any sudden permanent change in the sequence of DNA ŌŚ” Any agents that cause mutations are called ŌĆ£ MUTAGENŌĆÖS.ŌĆØ ŌŚ” Mutagens may be : I) chemical agents ex:1-4,dichlorobenzene 1-3, dichloro-2 propanol II) physical agents ex: by sun By microwaves By X-rays
- 4. INTRODUCTION ŌŚ” AMES TEST is widely employed method used to test whether a given chemical can cause mutations in DNA in test organism. ŌŚ” It is also called ŌĆ£BACTERIAL REVERSE MUTATION ASSAYŌĆØ. ŌŚ” It is based on the Principle of back mutation or reverse mutation.
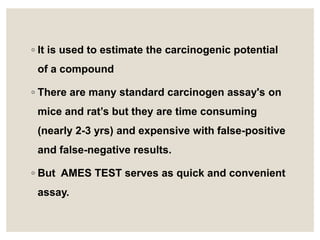
- 5. ŌŚ” It is used to estimate the carcinogenic potential of a compound ŌŚ” There are many standard carcinogen assay's on mice and ratŌĆÖs but they are time consuming (nearly 2-3 yrs) and expensive with false-positive and false-negative results. ŌŚ” But AMES TEST serves as quick and convenient assay.
- 6. HISTORY ŌŚ” AMES test was brought forwarded by BRUCE AMES in 1970. ŌŚ” He is a professor in biochemistry department in the University of California. ŌŚ” He developed this method as the previous methods or assayŌĆÖs are expensive and time consuming.
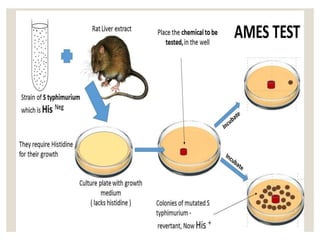
- 7. GENERAL PROCEDURE ŌŚ” Several strains of bacterium Salmonella typhimurium, carries mutations in genes involving in histidine(amino acid) synthesis. ŌŚ” The strains are auxotrophic mutants h- (defective organism or deficiency mutant) i.e., they require histidine for growth as they cannot produce it. ŌŚ” Ames test tests the capability of the test substance creating mutations that results in prototrophic state h+ i.e., the strains can grow on the histidine free medium.
- 8. Isolate an auxotrophic S.thypimurium for histidine Prepare a test suspension(-H) in a plain buffer and add small amounts of histidine. [NOTE :small amount of histidine is necessary of initial growth of bacteria] Also prepare (-H) control suspension i.e., without test chemicals. Incubate the suspensions at 37┬░C for 20mins.
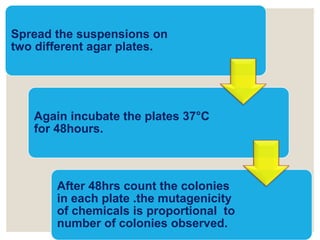
- 9. Spread the suspensions on two different agar plates. Again incubate the plates 37┬░C for 48hours. After 48hrs count the colonies in each plate .the mutagenicity of chemicals is proportional to number of colonies observed.
- 12. ŌŚ” Examples of chemicals that gives positive responses to Ames test : ’üČ 2-aminofluorene ’üČ Ethylene dibromide(EDC) ’üČ Ziram ’üČ Safrole ’üČ Saccharine ’üČ Aflotoxin
- 13. AMES TEST & CARCINOGENS ŌŚ” Mutagens identified via Ames test are possible carcinogens, early studies showed 90% of known carcinogens. ŌŚ” Later studies showed 50-70% carcinogens.
- 14. ŌŚ” The dose response curve obtained in Ames test is almost always LINEAR ,indicates that there is no threshold concentrations for Mutagenesis => indicates there may be no safe threshold level for chemical mutagens or carcinogens.
- 15. IMPORTANCE ŌŚ” Ames test is one of the test which screens for potential chemical carcinogens. ŌŚ” Under PESTICIDE ACT (USA), there are 8 tests and Ames test is one of them. ŌŚ” Similarly, TOXIC SUBSTANCES CONTROL ACT (USA). There are 6 tests and Ames test is also one of them.
- 16. LIMITATIONS ŌŚ” Salmonella typhimurium is a bacterium and thus not a perfect model of human body(which is why liver enzymes are added to the test) ŌŚ” In vitro model of eukaryotic cells has been adapted like yeast and mammalian cells grown in culture.
- 18. ŌŚ”Why are liver extracts are used in AMES TEST ?
- 19. Why are liver extracts are used in AMES TEST ? ŌŚ”Liver enzymes may activate some innocuous compounds, making them mutagenic
- 20. ŌŚ”WHICH BACTERIA GROW ON THE CULTURE PLATE IF AMES TEST IS POSITIVE?
- 21. WHICH BACTERIA GROW ON THE CULTURE PLATE IF AMES TEST IS POSITIVE? ŌŚ” his+ prototroughs the bacteria used in the Ames test to evaluate mutagenicity are his ŌĆō auxotrophs. If the Ames test is positive, these bacteria have reverted back to wild type and are his+ prototrophs.







![Isolate an auxotrophic S.thypimurium for
histidine
Prepare a test suspension(-H) in a plain
buffer and add small amounts of histidine.
[NOTE :small amount of histidine is
necessary of initial growth of bacteria]
Also prepare (-H) control suspension i.e.,
without test chemicals.
Incubate the suspensions at 37┬░C for
20mins.](https://image.slidesharecdn.com/amestest-180809104841/85/Ames-test-8-320.jpg)