Amino acid pathway
- 1. Amino Acid Pathway Presented by Sonali S Gadge P R Patil Institute of Pharmacy, Talegaon (SP), Dist- Wardha.
- 2. INTRODUCTION âĒ Amino acids are organic compounds containing amine (-NH2) and carboxyl (-COOH) functional groups, along with a side chain (R) group specific to each amino acid. âĒ Many amino acids contain only carbon, hydrogen, oxygen and nitrogen, but other atoms may be present (e.g. sulphur in cystine, and iodine in thyroxin).As already mentioned, more than one amino group may be present (e.g. Lysine, diaminocaproic acid) and more than one carboxylic acid group (e.g. aspartic or amino succinic acid). âĒ Some amino acids are aromatics such as phenylalanine, or heterocyclic such as proline (pyrolidine nucleus), tryptophan (indole nucleus) and histidine (imidazole nucleus).
- 3. Properties âĒ Amino acids are generally soluble in water but only slightly soluble in alcohol. A general test is to warm with ninhydrin, when, with the exception of proline, which gives a yellow, they give a pink, blue or violet colour. âĒ Amino acids do not respond to the biuret test (compare polypeptides and proteins). âĒ Certain amino acids are detected by more specific tests (e.g. histidine gives colour reactions with diazonium salts).
- 4. Classification 1. Nonpolar/Hydrophobic amino acids: Glycine, Alanine, Valine, leucine, Methionine, Phenylalanine, Proline. 2. Polar/hydrophilic amino acids: Serine, Cysteine, Tyrosine, Glutamic acid, Asparatic acid, Lysine, Arginine. 3. Sulphur Containing amino acids: Methionine, Cysteine
- 5. Essential and Non-essential amino acids in Humans Essential amino acids Non-essential amino acids Arginine Alanine HIstidine Asparagine Isoleucine Aspartate Lysine Cysteine Methionine Glutamate Phenylalanine Glutamine Threonine Glycine Tryptophan Proline Valine Serine Leucine Tyrosine
- 6. Amino acids found in proteins âĒ These include -alanine; arginine; asparagine(amide of aspartic acid), abundant in many plants, particularly etiolated seedlings aspartic acid; amino succinic acid, involved in the biosynthesis of purines ; cysteine, which contains sulphur; cystine or dicysteine (in hair and insulin); 3,5-di-iodotyrosine (in thyroid); glutamic acid (a component of the folic acid vitamins); glutamine (free in animals and plants, e.g. Sugar beet); glycine (aminoacetica cid); histidine;6 -hydroxylysine( in gelatin); hydroxyproline (in gelatin); leucine (cx- aminocaproic acid); isoleucine; lysine; methionine (contains a sulphur atom); 3-monoiodotyrosine (in thyroid); phenylalanine; proline; serine (in phosphoproteins such as casein);threonine( in casein); thyroxin (theiodine-containing thyroid- hormone);3,5,3ltriiodothyronine (in thyroid); tryptophan; tyrosine; and valine.
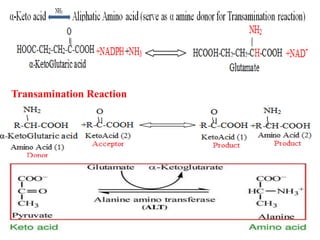
- 7. Amino acid pathway âĒ Amino acid synthesis is the set of biochemical processes (Metabolic pathway) by which the amino acids are produced. âĒ All amino acids are derived from the intermediates in glycolysis, the Citric Acid Cycle, or Pentose Phosphate Pathway. âĒ Nitrogen enters the pathway by way of Glutamate and Glutamine. âĒ Organisms vary greatly in their ability to synthesize the 20 common amino acids. âĒ Whereas most bacteria and plants can synthesize all 20, mammals can synthesize about half of them- generally those with simple pathways. The non-essential amino acids not needed in the diet. âĒ The remaining, the essential amino acids, must be obtained from food.
- 8. âĒ Amino acids are the precursors of some secondary metabolite (Eg. Alkaloids). âĒ Most amino acids are found in nature â Îą amino acids. âĒ Amino acid pathway starts from Glycolysis, Krebâs cycle (TCA Cycle) â Branched from its intermediates. âĒ Plant synthesize all 20 amino acids (Aliphatic, Aromatic Heterocyclic). âĒ Nitrogen enters metabolic reaction by Reductive amination.