Amino acids structure
- 1. Amino Acids Structure & Properties By Dr. Narottam Agrawal Faculty, Rajkiya Medical College Jalaun (Orai), U.P. INDIA
- 2. Specific Learning Objectives (SLO) ï General structure of amino acid ï Amino acid as Ampholytes (acid and base), Zwitter ions. ï Classification of amino acid on the basis of side chain, chemical composition, Nutritional Requirement and metabolic fate. ï Derived amino acids. ï Optical properties of amino acids. ï Acid-Base properties and Buffer characteristic. ï Biological Important Peptides ï Proteins based on nutritional value
- 3. STRUCTURE OF THE AMINO ACID Each amino acid (except for proline) has: 1. A carboxyl group (-COO-) 2. An amino group (-NH3 +) 3. Side chain ("R-group") bonded to the Îą-carbon atom. ï Carboxyl group of one amino acid joined together with amino group of second amino acid by peptide linkage and a dipeptide is formed.
- 4. Nonionic and zwitterion forms of amino acids The zwitterion predominates at neutral pH Week acid Week base
- 5. Classification of Amino Acids on the basis of side chains Amino acids varies in their side chains, thatâs why they are classified on the basis of their side chain. Amino acids are divided in to five groups. They are- ï Amino acids with nonpolar aliphatic side chains. ï Amino acids with Aromatic R Group. ï Amino acids with uncharged polar side chains. ï Amino acids with Positively Charged R Groups (Basic amino acids). ï Amino acids with Negatively charged R Groups (Acidic amino acids).
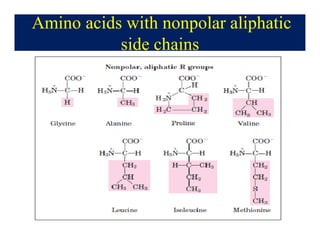
- 6. Amino acids with nonpolar aliphatic side chains
- 7. Amino acids with nonpolar aliphatic side chains âĒ The side chains of amino acids cluster in the interior of the protein due to hydrophobicity. âĒ The side chain of proline and its Îą-amino group form a ring structure. âĒ Proline gives the fibrous structure of collagen, and interrupts the Îą-helices found in globular proteins.
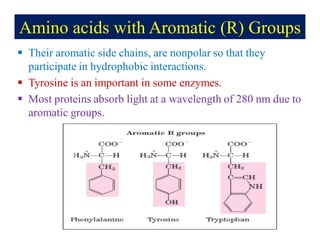
- 8. Amino acids with Aromatic (R) Groups ï§ Their aromatic side chains, are nonpolar so that they participate in hydrophobic interactions. ï§ Tyrosine is an important in some enzymes. ï§ Most proteins absorb light at a wavelength of 280 nm due to aromatic groups.
- 9. Absorption Spectra of Aromatic Amino Acids This property exploited by researchers in the characterization of proteins.
- 10. Amino acids with uncharged polar side chains More hydrophilic because they form hydrogen bonds with water. They are serine, threonine, cysteine, asparagine, and glutamine.
- 11. âĒ Cysteine contains a sulfhydryl group (-SH), an important component of the active site of many enzymes. âĒ Two cysteines can become oxidized to form a dimmer cystine, which contains a covalent cross-link called a disulfide bond (-S-S-). âĒ Serine and threonine contain a polar hydroxyl group. This group serve as a site of attachment (in enzymes) for groups such as a phosphate. âĒ Amide group of asparagine, as well as the hydroxyl group of serine or threonine serve as a site of attachment for oligosaccharide chains in glycoproteins. Amino acids with uncharged polar side chains
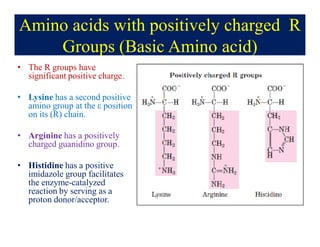
- 12. Amino acids with positively charged R Groups (Basic Amino acid) âĒ The R groups have significant positive charge. âĒ Lysine has a second positive amino group at the Îĩ position on its (R) chain. âĒ Arginine has a positively charged guanidino group. âĒ Histidine has a positive imidazole group facilitates the enzyme-catalyzed reaction by serving as a proton donor/acceptor.
- 13. Amino acids with Acidic Side Chains ïķAspartic and glutamic acid are proton donors. ïķAt neutral pH, the side chains of these amino acids are fully ionized. ïķAt low pH, the side chains of these amino acids are fully protonated. ïķThey have a negatively charged carboxylate group (-COO-) at physiologic pH.
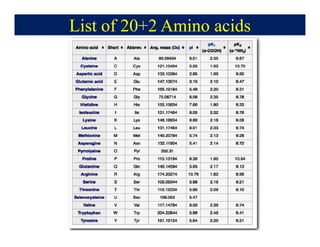
- 14. List of 20+2 Amino acids
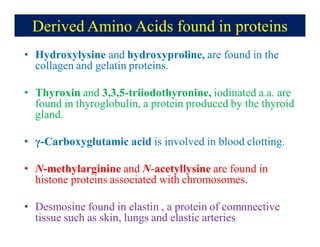
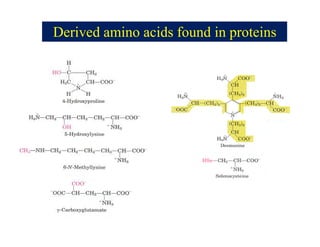
- 15. Derived Amino Acids found in proteins âĒ Hydroxylysine and hydroxyproline, are found in the collagen and gelatin proteins. âĒ Thyroxin and 3,3,5-triiodothyronine, iodinated a.a. are found in thyroglobulin, a protein produced by the thyroid gland. âĒ Îģ-Carboxyglutamic acid is involved in blood clotting. âĒ N-methylarginine and N-acetyllysine are found in histone proteins associated with chromosomes. âĒ Desmosine found in elastin , a protein of comnnective tissue such as skin, lungs and elastic arteries .
- 16. Derived amino acids found in proteins
- 18. Classification Based on Chemical Composition Small amino acids â Glycine, Alanine Branched amino acids â Valine, Leucine, Isoleucine Hydroxy amino acids (-OH group) â Serine, Threonine Sulfur amino acids â Cysteine, Methionine Aromatic amino acids â Phenylalanine, Tyrosine, Tryptophan Acidic amino acids and their derivatives â Aspartate, Asparagine, Glutamate, Glutamine Basic amino acids â Lysine, Arginine, Histidine Imino acid - Proline
- 19. âĒ Required in diet âĒ Humans incapable of forming requisite carbon skeleton (FILTH RKM VW) Arginine* Histidine* Isoleucine Leucine Valine Lysine Methionine Threonine Phenylalanine Tryptophan * Essential in children, not in adults Essential Amino Acids in Humans
- 20. Amino acid based on Metabolic Fate On the basis of their end products amino acids are following types:- âĒ Ketogenic amino acids: They are degraded to Acetyl Co A or Acetoacetyl Co A. eg: Leucine and lysine âĒ Ketogenic and glucogenic amino acids : eg: Tryptophan, Isoleucine, phenylalanine and tyrosine (WIFY). âĒ Glucogenic amino acids : There are degraded to pyruvate, Îą- ketoglutarate, succinyl-coA, fumarate or oxalloacetate. Eg: Rest of the amino acids ïķ All these intermediate products enter in to citric acid cycle and used for â - Synthesis of glucose - Synthesis of lipid - Production of energy and converted in to CO2 and H2O
- 21. Metabolic fate of amino acids
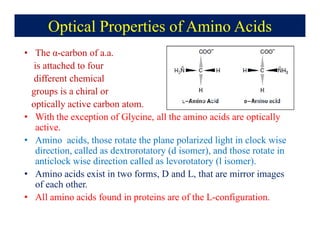
- 22. Optical Properties of Amino Acids âĒ The Îą-carbon of a.a. is attached to four different chemical groups is a chiral or optically active carbon atom. âĒ With the exception of Glycine, all the amino acids are optically active. âĒ Amino acids, those rotate the plane polarized light in clock wise direction, called as dextrorotatory (d isomer), and those rotate in anticlock wise direction called as levorotatory (l isomer). âĒ Amino acids exist in two forms, D and L, that are mirror images of each other. âĒ All amino acids found in proteins are of the L-configuration.
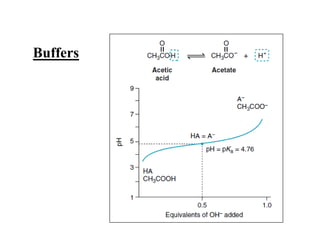
- 23. ACIDIC AND BASIC PROPERTIES OF AMINO ACIDS ïķAmino acids in aqueous solution contain weakly acidic Îą-carboxyl groups and weakly basic Îą-amino groups. ïķ Each of the acidic and basic amino acids contains an ionizable group in its side chain. ïķ Thus, both free and some of the combined amino acids in peptide linkages can act as buffers. ïķThe concentration of a weak acid (HA) and its conjugate base(A-) is described by the Henderson-Hasselbalch equation.
- 24. Derivation of the equation âĒ For the reaction (HA H+ + A- ) [H+] [A-] âĒ Ka = âââââ ------ (1) [HA] âĒ By solving for the [H+] in the above equation, taking the logarithm of both sides of the equation, multiplying both sides of the equation by -1, and substituting pH = -log [H+] and pKa = -log [Ka] we obtain: [A-] âĒ pH = pKa + log âââ ------ (2) [HA] It is the (Henderson-Hasselbalch equation)
- 25. Protonated form Unprotonated form (conjugate base) HA H+ A- + Ka [HA] [H+] [A-] = = Ka x [A-] [HA] [H+] -log [H+] -log Ka -log [A-] [HA] = pH = pKa log [A-] [HA] + Henderson/Hasselbach equation and pKa
- 26. Buffers
- 27. Titration of an amino acid At Isoelectric point Zwitter ion is found which has net Charge Zero
- 28. Titration curve of Glycine
- 29. Titration curve of Acidic Amino acid: Glutamate
- 30. Titration curve of Basic Amino acid: Histidine
- 31. Biologically Important Peptides Some oligopeptides have biological importance these are- Thyrotropin releasing hormone (TRH):- made up of tripeptide (Glu-His-Pro) released from hypothalamus and act on ant. Pituitary for release of thyrotropin (TSH) and prolactin. Glutathione :- It is a tripeptide Gammaglutamyl-cysteinyl-glycine, involve in RBC membrane integrity and important for keeping enzyme in active state. Enkephalin: Pentapeptide, act as neurotransmitter in brain. Oxytocin & Vasopressin (ADH):- Both are nanopeptides secreted by posterior pituitary Angiotensin :- Angiotensin I contain 10 amino acids and Angiotensin II has 8 amino acids they increase blood pressure.
- 32. Protein based on Nutrition value Nutrionally Rich Proteins:- They are complete proteins or first class proteins. Contains all the essential amino acids in the required proportion. On supplying these protein in diet, children will grow satisfactorily (eg. Casein of milk) Incomplete protein :- They lack one essential amino acid. They can not promote body growth in children but may be able to sustain body weight in adults. (eg. proteins from pulses are lack of methionine, protein of cereals lack in lysine , but combination of both adequate growth may be obtained. Poor Proteins :- They lack in many essential amino acids and diet based on these protein will not sustain the original body weight (Zein from corn lacks Tryptophan and Lysin )
- 33. Summary âĒ The 20 amino acids commonly found as residues in proteins contain an Îą-carboxyl group, an Îą-amino group, and a distinctive R group substituted on the Îą-carbon atom. âĒ The Îą-carbon atom of all amino acids except glycine is asymmetric, and thus amino acids can exist in at least two stereoisomeric forms. âĒ Only the L stereoisomers, are found in proteins, Since active site of enzymes are assymetric so they produce L-AMINO ACIDS. âĒ Amino acids are classified into five types on the basis of the polarity and charge (at pH 7) of their R groups. âĒ Amino acids vary in their acid-base properties and have characteristic titration curves. Monoamino monocarboxylic amino acids (with nonionizable R groups) are diprotic acids (+H3NCH(R)COOH) at low pH and exist in several different ionic forms as the pH is increased. Amino acids with ionizable R groups have additional ionic species, depending on the pH of the medium and the pKa of the R group.
- 34. Thank youâĶ..

















![Derivation of the equation
âĒ For the reaction (HA H+ + A- )
[H+] [A-]
âĒ Ka = âââââ ------ (1)
[HA]
âĒ By solving for the [H+] in the above equation,
taking the logarithm of both sides of the
equation, multiplying both sides of the equation
by -1, and substituting pH = -log [H+] and pKa
= -log [Ka] we obtain:
[A-]
âĒ pH = pKa + log âââ ------ (2)
[HA]
It is the (Henderson-Hasselbalch equation)](https://image.slidesharecdn.com/aminoacidsstructure-210419150937/85/Amino-acids-structure-24-320.jpg)
![Protonated form Unprotonated form (conjugate base)
HA H+ A-
+
Ka
[HA]
[H+] [A-]
=
= Ka x
[A-]
[HA]
[H+]
-log
[H+]
-log Ka
-log
[A-]
[HA]
=
pH = pKa log
[A-]
[HA]
+
Henderson/Hasselbach equation and pKa](https://image.slidesharecdn.com/aminoacidsstructure-210419150937/85/Amino-acids-structure-25-320.jpg)