Anti infective agent, Classification and reaction.pptx
- 2. Anti-Infective Agents ŌĆó Anti-infective agents are drugs that prevent or treat infections by inhibiting the growth of or killing microorganisms such as bacteria, viruses, fungi, and parasites. ŌĆó These agents act at the site of application, limiting systemic absorption and minimizing side effects. ŌĆó Antiseptics: Antiseptics are chemical agents applied to living tissues (such as skin, mucous membranes, or wounds) to inhibit the growth of or destroy microorganisms, preventing infection without causing significant harm to the tissue. ŌĆó Example: Povidone-iodine, Hydrogen peroxide, Alcohol. ŌĆó Disinfectants: Disinfectants are chemical agents used on non-living surfaces and objects to destroy or inactivate microorganisms, including bacteria, viruses, and fungi. They are not safe for application to living tissues due to their higher toxicity. ŌĆó Example: Sodium hypochlorite (bleach), Phenol.
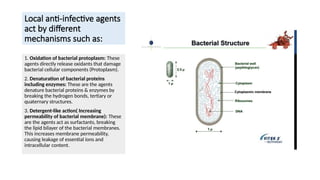
- 3. Local anti-infective agents act by different mechanisms such as: 1. Oxidation of bacterial protoplasm: These agents directly release oxidants that damage bacterial cellular components (Protoplasm). 2. Denaturation of bacterial proteins including enzymes: These are the agents denature bacterial proteins & enzymes by breaking the hydrogen bonds, tertiary or quaternary structures. 3. Detergent-like action( Increasing permeability of bacterial membrane): These are the agents act as surfactants, breaking the lipid bilayer of the bacterial membranes. This increases membrane permeability, causing leakage of essential ions and intracellular content.
- 4. Classification Alcohols and related compounds: Alcohol, Isopropyl alcohol, Formaldehyde solution Phenols and their Derivatives: p-Chlorophenol, Hexachlorophene, Hexyl resorcinol Oxidizing agents: Hydrogen peroxide solution, Hydrous benzoyl peroxide Halogen containing compounds: Iodine tincture, Chlorine containing compound- Halazone Cationic surfactants: Benzalkonium chloride, Cetylpyridinium chloride Dyes: Genetian Violet, Methylene blue Nitrogen compounds: Nitrofurazone, Furazolidone
- 5. Alcohols and related compounds: ŌĆó Alcohols are widely used antiseptics, especially during dressing of wounds. ŌĆó These act by causing denaturation of proteins. ŌĆó Ethyl and isopropyl alcohols in the con. of 30-70% are used as antiseptics. ŌĆó Structure Activity Relationship (SAR): ŌĆó The activity of alcohols increases with increase in molecular weight and chain length. ŌĆó The potency of aliphatic alcohols increases with lipophilicity. ŌĆó Branching and addition of hydroxy groups decrease the potency.
- 6. Alcohols and related compounds: ŌĆó Ethyl Alcohol (Ethanol): CHŌéāCHŌééOH ŌĆó Chemical Formula: CŌééHŌéģOH ŌĆó Molecular Weight: 46.07 g/mol ŌĆó Appearance: Clear, colorless liquid ŌĆó Used in hand sanitizers, pre-injection skin cleaning, and minor wound care. ŌĆó Used to disinfect surfaces in hospitals, laboratories, and pharmaceutical industries.
- 7. Isopropyl alcohol ŌĆó Isopropyl alcohol, also known as isopropanol or 2-propanol, is a widely used antiseptic, disinfectant, and solvent. It is effective against bacteria, viruses, and fungi, making it a common component in hand sanitizers and surface disinfectants. ŌĆó Chemical Formula: (CHŌéā)ŌééCHOH ŌĆó Molecular Weight: 60.1 g/mol ŌĆó Appearance: Clear, Colorless liquid
- 8. Formaldehyde ŌĆó Formaldehyde is a simple organic compound with strong disinfectant and preservative properties. It is widely used in healthcare, laboratories, and industries for sterilization and preservation purposes. ŌĆó Chemical Formula: CHŌééO ŌĆó Molecular Weight: 30.03 g/mol ŌĆó Appearance: Colorless gas or liquid ŌĆó The structure consists of a carbonyl group (C=O) bonded to two hydrogen atoms ŌĆó Mechanism of Action: Formaldehyde cross-links with proteins and nucleic acids, disrupting cell function and leading to microbial death.
- 9. Phenols and their Derivatives: Phenols and their derivatives have wide antibacterial properties and antiseptics. They act as germicidals by denaturing the bacterial proteins. Structure ŌĆō Activity Relationship ( SAR): ŌĆó The activity of phenols is related to no. of free hydroxy groups. ŌĆó Halogenation at para position to hydroxy group potentials the activity ŌĆó The antibacterial activity of alkyl phenols increase with increase in size of the alkyl group.
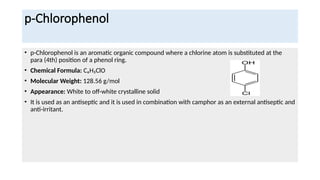
- 10. p-Chlorophenol ŌĆó p-Chlorophenol is an aromatic organic compound where a chlorine atom is substituted at the para (4th) position of a phenol ring. ŌĆó Chemical Formula: CŌéåHŌéģClO ŌĆó Molecular Weight: 128.56 g/mol ŌĆó Appearance: White to off-white crystalline solid ŌĆó It is used as an antiseptic and it is used in combination with camphor as an external antiseptic and anti-irritant.
- 11. Hexachlorophene Chemical formula: C13H6Cl6O2 2,2 methylene- bis (3,4,6- trichlorophenol) Hexachlorophene consists of 2 trichlorophenol (C6H2Cl3OH) rings connected by methylene (-CH2-) bridge at the second carbon of each ring It is a white crystaline powder and insoluble in water. It is mainly used in soaps, detergents, creams and emulsions for tropical application.
- 12. Synthesis Formaldehyde activation under acidic medium producing a reactive electrophilic intermediate. Electrophilic carbon from formaldehyde reacts with ortho positions of the 2,4,5- trichlorophenol. Methylene Methylene Bridge Formation: A second molecule of trichlorophenol reacts, completing the formation of the bisphenol structure (hexachlorophene).
- 13. Hexyl resorcinol ŌĆó Hexylresorcinol is an organic compound that belongs to the resorcinol family, known for its antiseptic and anesthetic properties. It is commonly used in pharmaceutical and personal care products. ŌĆó IUPAC Name: 4-Hexyl resorcinol ŌĆó Molecular Formula: CŌéüŌééHŌéüŌéłOŌéé ŌĆó It occurs as white, needle shape crystals with a faint odour. ŌĆó It is freely soluble in water. ŌĆó It is odourless and nonstaining.
- 14. Synthesis:
- 15. Oxidizing agents: ŌĆó Oxidising agents have germicidal property and are used in treatment of infections caused by anaerobic organisms( donŌĆÖt require oxygen to grow). ŌĆó The most common oxidising agents are Hydrogen peroxide, Potassium permanganate, Zinc peroxide, Hydrousbenzoyl peroxide.
- 16. Hydrogen peroxide (H2O2) ŌĆó Hydrogen peroxide is a good disinfectant and sterilant. It is rapidly decomposes releasing nascent oxygen which produces antibacterial action. ŌĆó It is not stable and may undergo decomposition on storage. ŌĆó It is used in the form of carbamate peroxide which releases hydrogen peroxide when come into contact with water. ŌĆó It is mainly used as ear drops to remove slough, ear wax etc. ŌĆó Hydrous Benzoyl Peroxide: It is used topically in the therapy of mild to moderate acne vulgaris and to removes dead skin cells.
- 17. Halogen containing compounds ŌĆó Iodine tincture: 2% solution of iodine in alcohol. It is effective over a wide pH range and the bactericidal activity is related to the amount of free iodine generated. ŌĆó Iodine acts by oxidising tyrosine and sulfhydryl groups in proteins causing inactivation.
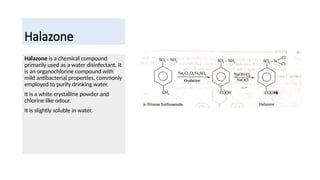
- 18. Halazone ŌĆó Halazone is a chemical compound primarily used as a water disinfectant. It is an organochlorine compound with mild antibacterial properties, commonly employed to purify drinking water. ŌĆó It is a white crystalline powder and chlorine like odour. ŌĆó It is slightly soluble in water.
- 19. Halazone Halazone is a chemical compound primarily used as a water disinfectant. It is an organochlorine compound with mild antibacterial properties, commonly employed to purify drinking water. It is a white crystalline powder and chlorine like odour. It is slightly soluble in water.
- 20. Bacteria ŌĆó Gram-Positive Bacteria ŌĆó Thick (20-80 nm) ŌĆó Outer Membrane: Absent ŌĆó Lipid Content: Low ŌĆó Gram Staining Result: Retains crystal violet (purple/blue) ŌĆó Gram-Negative Bacteria ŌĆó Thin (5-10 nm) ŌĆó Present ŌĆó High ŌĆó Loses crystal violet; stains with safranin (pink/red)
- 21. Cationic Surfactants ŌĆó Cationic surfactants are effective against a number of Gram-possetive and Gram-negative bacteria, fungi and viruses. ŌĆó These cations are absorbed onto the surface of the bacteria resulting in cell membrane disruption, protein denaturation and enzyme inhibitions. ŌĆó The detergent property also helps in cleaning dirty wounds. ŌĆó These compounds have the advantage of having low toxicity, non-staining, high water solubility.
- 22. Benzalkonium Chloride (BAK) ŌĆó Benzalkonium Chloride is a widely used quaternary ammonium compound (QAC) with antimicrobial, antiseptic, and surfactant properties. It is commonly found in disinfectants, hand sanitizers, eye drops, and pharmaceutical formulations. ŌĆó Chemical Formula: CŌééŌéćHŌéäŌééClN (for dodecylbenzyl version) ŌĆó General Formula: CŌéåHŌéģCHŌééNŌü║(R)ŌéāClŌü╗ ŌĆó Where: ŌĆó CŌéåHŌéģCHŌéé ŌĆō Benzyl group ŌĆó NŌü║(R)Ōéā ŌĆō Quaternary ammonium (R = long alkyl chain, typically dodecyl (12-carbon alkyl chain) or tetradecyl) ŌĆó IUPAC Name ŌĆó N-benzyl-N,N-dimethyldodecan-1-aminium chloride
- 23. Dyes: Gentian Violet Structure: Gentian violet consists of a triphenylmethane core with three dimethylamino (ŌłÆN(CHŌéā)Ōéé) groups attached to the phenyl rings and a chloride ion. The central carbon is bonded to three phenyl rings, each substituted with a dimethylamino group. IUPAC: [4-(Dimethylamino)phenyl]-bis[4- (dimethylamino)phenyl]methylium chloride Soluble in water and ethanol Gentian violet with antiseptic, antifungal, and staining properties. It is commonly used in microbiology, medicine,
- 24. Nitrogen compounds Nitrofurazone is an antimicrobial agent primarily used for the treatment of wounds, burns, and bacterial infections. It belongs to the nitrofuran class of drugs, known for their broad-spectrum antibacterial properties. Nitrofurazone consists of a furan ring substituted with a nitro group (ŌłÆNOŌéé) and a hydrazone moiety. The furan ring (CŌéäHŌéāO) carries a nitro group at position 5, and the hydrazone group is attached at position 2. IUPAC Name N-(5-Nitro-2-furfurylidene)-1-aminohydantoin Topical Antibacterial Agent ŌĆō Used in ointments for skin infections, burns, and wounds.




















![Dyes:
Gentian Violet
Structure: Gentian violet consists of a triphenylmethane core
with three dimethylamino (ŌłÆN(CHŌéā)Ōéé) groups attached to the
phenyl rings and a chloride ion.
The central carbon is bonded to three phenyl rings, each
substituted with a dimethylamino group.
IUPAC:
[4-(Dimethylamino)phenyl]-bis[4-
(dimethylamino)phenyl]methylium chloride
Soluble in water and ethanol
Gentian violet with antiseptic, antifungal, and staining
properties. It is commonly used in microbiology, medicine,](https://image.slidesharecdn.com/medicinalchem-250110114619-8b3f821f/85/Anti-infective-agent-Classification-and-reaction-pptx-23-320.jpg)































































