Apoptosis
- 1. MODERATOR DR. POONAM MAâAM LECTURER PATHOLOGY DEPTT. MRAMC PRESENTED BY : ABHISHEK KUMAR YADAV R.NO. - 04
- 2. oIntroduction oEtiopathogenesis oMorphological, Biochemical changes oMechanism oIntrinsic pathway oExtrinsic pathway oDisorders of apoptosis oConclusion
- 3. INTRODUCTION
- 4. Apoptosis - Definition A pathway of cell death induced by a tightly regulated suicidal program, in which the cells destined to die activate enzymes that degrade cells own nuclear DNA and nuclear, cytoplasmic proteins.
- 5. Significance of apoptosis âĒ During development â many cells produced in excess â programmed cell death â contribute to sculpturing of organs & tissues. âĒ In human body about one lakh cells are produced every second by mitosis and a similar number die by apoptosis.
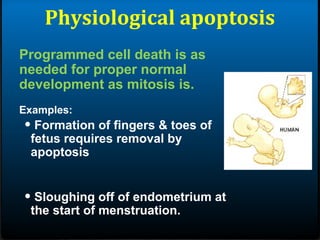
- 7. Physiological apoptosis Programmed cell death is as needed for proper normal development as mitosis is. Examples: âĒ Formation of fingers & toes of fetus requires removal by apoptosis âĒ Sloughing off of endometrium at the start of menstruation.
- 8. Apoptosis in physiologic situations ïą Programmed destruction during embryogenesis ïą Involution of hormone dependent tissues ïą Cell loss in proliferating cell populations ïą Elimination of harmful self- reactive lymphocytes ïą Death of host cells
- 9. Apoptosis: in embryogenesis Morphogenesis (eliminates excess cells): Selection (eliminates non-functional cells):
- 10. Apoptosis: importance in adults Tissue remodeling (eliminates cells no longer needed): Virgin mammary gland Late pregnancy, lactation Involution (non-pregnant, non-lactating) Apoptosis Apoptosis - Testosterone Prostate gland
- 11. Apoptosis: in immunity Immunity (eliminates dangerous cells): Self antigen recognizing cell Organ size (eliminates excess cells):
- 12. Apoptosis in pathological conditions - DNA damage - Accumulation of misfolded proteins - Cell death in certain infections - Pathological atrophy in parenchymal organs
- 14. CLASSIC CHANGES âĒ Cell shrinkage âĒ Nuclear fragmentation âĒ Chromatin condensation âĒ Chromosomal DNA fragmentation âĒ Formation of cytoplasmic blebs& apoptotic bodies âĒ Phagocytosis
- 17. STAGES OF CLASSIC APOPTOSIS Healthy cell DEATH SIGNAL / STIMULI (extrinsic or intrinsic) Commitment to die (reversible) EXECUTION (irreversible) Dead cell (condensed, crosslinked) ENGULFMENT (macrophages, neighboring cells) DEGRADATION
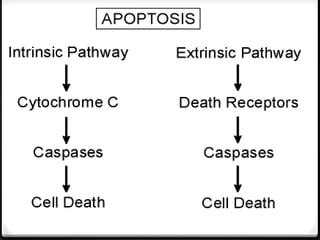
- 19. Initiation of apoptosis by activation of signalling pathways : There are two main signalling pathways in apoptosis : (A) Extrinsic/death receptor-initiated pathway :
- 21. Activation of caspase cascade Release of several mt proteins (B) INTRINSIC/MITOCHONDRIAL PATHWAY :
- 22. °ä°ŋą·°ÕķŲâĶ
- 24. Execution Phase
- 25. HISTOLOG Y
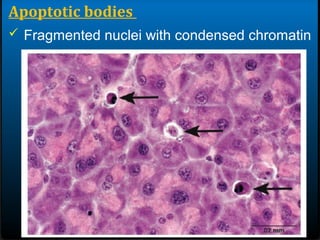
- 26. Apoptotic bodies ïž Round oval mass of intensely eosinophilic cytoplasm
- 27. Apoptotic bodies ïž Fragmented nuclei with condensed chromatin
- 28. REGULATION OF APOPTOSIS âĒ Release of mitochondrial pro-apoptotic proteins tightly controlled by BCL2 family of proteins. âĒ Antiapoptotic proteins : BCL2, BCLXL & MCL1 âĒ Proapoptotic proteins : BAX and BAK âĒ BCL2 sensor proteins : BAD, BIM, BID, Puma, Noxa (also called BH3 proteins) âĒ Also, cytoplasm of normal cells contains inhibitors of apoptosis (IAP) which are neutralized by proapoptotic factors.
- 31. Apoptosis: Role in Disease TOO MUCH: Tissue atrophy TOO LITTLE: Hyperplasia Neurodegeneration Thin skin etc Cancer Athersclerosis etc
- 32. Neurodegeneration âNeurons are post-mitotic. âNeuronal death caused by loss of proper connections, loss of proper growth factors (e.g. NGF), and/or damage (especially oxidative damage). âNeuronal dysfunction or damage results in loss of synapses or loss of cell bodies (synaptosis, can be reversible; apoptosis, irreversible) âPARKINSON'S DISEASE âALZHEIMER'S DISEASE âHUNTINGTON'S DISEASE etc.
- 34. CANCER ï Apoptosis eliminates damaged cells (damage => mutations => cancer) ï Tumor suppressor p53 controls senescence and apoptosis responses to damage. ï Most cancer cells are defective in apoptotic response(damaged, mutant cells survive) ï High levels of anti-apoptotic proteins or ï Low levels of pro-apoptotic proteins ===> CANCER
- 35. CANCER VIRUS ASSOCIATED CANCER âĒ Human papilloma viruses (HPV) âĒcauses cervical cancer âĒproduces a protein (E6)-binds & inactivates apoptosis promoter p53. âĒ Epstein-Barr Virus (EBV) - cause of mononucleosis and a/w some lymphomas âproduces a protein similar to Bcl-2 âproduces another protein that causes the cell to increase its own production of Bcl-2. Both these actions make the cell more resistant to apoptosis (thus enabling a cancer cell to continue to proliferate).
- 36. âĒ Some B-cell leukemia and lymphomas express high levels of Bcl-2 â block apoptotic signals. The high levels result from a translocation of BCL-2 gene into an enhancer region for antibody production. âĒ Melanoma cells avoid apoptosis by inhibiting expression of the gene encoding Apaf-1. CANCER
- 37. ConClusion
- 38. DAMAGE Physiological death signals DEATH SIGNAL PROAPOPTOTIC PROTEINS (dozens!) ANTIAPOPTOTIC PROTEINS (dozens!) DEATH