Assignment presentation data logging
- 1. DATA-LOGGING ACID-BASE TITRATION USING PH SENSOR NAME: KESAVARTINII A/P BALA KRISNAIN (D20101039444) CYPRIANA CAYAUS (D20101037509) BRENDA MITCHELLE MORRIS (D20101037527 ) GROUP : A PROGRAMME : PENDIDIKAN SAINS SEMESTER :5 LECTURER : EN. AZMI BIN IBRAHIM
- 2. DATA LOGGING ’éó Theprocess where computer used to collect data through sensors, data analyzed and saved, and the results of the collection and analysis become the output. ’éó The data logger is the electronic instrument that records the measurement over time.
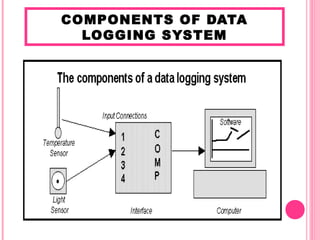
- 3. COMPONENTS OF DATA LOGGING SYSTEM
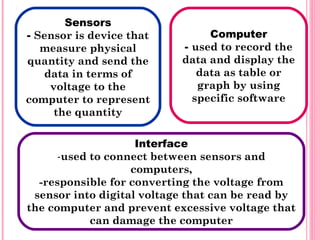
- 4. Sensors - Sensor is device that Computer measure physical - used to record the quantity and send the data and display the data in terms of data as table or voltage to the graph by using computer to represent specific software the quantity Interface -used to connect between sensors and computers, -responsible for converting the voltage from sensor into digital voltage that can be read by the computer and prevent excessive voltage that can damage the computer
- 5. ADVANTAGES AND DISADVANTAGES Advantages Disadvantages 1. New innovative 1. If the data logging experiments and equipment breaks down, dangerous some data could be lost experiments become and not recorded possible 2. Equipments needed in 2. More accurate result data logging are very as there is no expensive even if it is possibility of human just for a small task error 3. Caused loss of 3. Easier to understand conventional methods of scientific data collection and experimentation and reduced the practice of scientific concept, and graphing skills better graphing.
- 6. ENGAGE
- 7. What we know about acid and bases? Acids - Level of pH: 1 to 6 - Example: Lemons, orange juice and vinegar are examples of acids. Bases - Level of pH: 8 to 14 - Example: Mustards and medicines are examples of a base. Flavor is added to medicines to offset the bitter taste. Water - Level of pH: 7, Natural
- 8. EMPOWER ACID-BASE TITRATION USING PH SENSOR ’éó Planning and doing experiment
- 9. PLANNING AND CARRYING OUT EXPERIMENT Interface Data logger
- 10. RESULTS
- 11. FIRST DERIVATIVES OF DATA
- 12. QUESTIONS 1. What does the graph tell you about the amount of hydrochloric acid and sodium hydroxide needed for the titration? 2. What does the first derivatives graph represents? 3. Explain the difference between an endpoint and equivalence point in a titration. 4. Can we titrate a solution of unknown concentration with another solution of unknown concentration and still get a meaningful answer? Explain. 5. What is the chemical equation involved in this acid- base titration?
- 13. ENHANCE 1. Citric acid - Plays a role in one famous stomach remedy, or antacid. - Antacids are more generally associated with alkaline substances, used for their ability to neutralize stomach acid. Example : The fizz in Alka-Seltzer;
- 14. 2. Aluminum hydroxide -Is an interesting base, because it has a wide number of applications, including its use in antacids. - It reacts with and neutralizes stomach acid (Hydrochloric acid, HCl). Example: found in commercial antacids such as Di- GelŌäó, GelusilŌäó, and MaaloxŌäó(relief of heartburn and acid indigestion).
- 16. 3. Baking soda (sodium bicarbonate and sodium hydrogen) - Use in baking and other application such as in Alka- Seltzer (addition of citric acid to improve the flavour), fighting fires (smoothers flames by obstructing the flow of oxygen to the fire) - Baking Powder = Baking soda + weak acid = carbon dioxide (causes dough and batters to rise)
- 17. QUESTION : Why Stomach Acid might┬Āneed to be neutralized? ’āś substance inside the body that is able to digest the living tissue around it’āĀrequire some of it to be neutralized. ’āś when the acid leaks upward through a valve into the oesophagus, causing what is known as acid reflux or heartburn. ’āś heartburn is when the stomach acids get into the oesophagus and eat away at the lining, causing a burning sensation in the chest and throat. ’āś to reduce the leakage of stomach acid into the oesophagus, neutralization of some of the acid would be required, avoid the oesophagus receives permanent damage. In order to do this’āĀ antacids