AtomicTheory 05-31-07
- 1. Atomic Physics Prelude to Modern Science Dr Gary Stilwell
- 2. History and Discovery What are atoms Early models Problems to solve The modern Atom Some definitions Seeing the atom Structure of matter and force
- 3. Power of the Atom - E=mc 2 March 1, 1954 H-bomb test at Bikini Atol
- 4. Matter and Black Holes Later we will be discussing black holes. At the center of a black hole matter is squeezed into zero volume. Let us now discuss this matter. . .
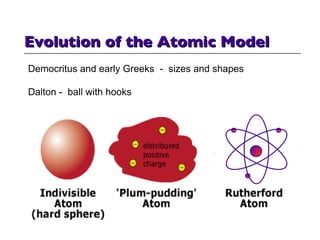
- 5. So, Just What Is This Matter that can be so Squeezed in a Black Hole? The Easy Answer is -- Atoms: Democritus (c. 430 BCE) a)/tomoj "By convention sweet is sweet . . . But in reality nothing exists but atoms and the void". What we perceive by the senses is not reality! Sizes & shapes determine material properties. - - - Pierre Gassendi (ca. 1624) atoms not exist forever, made by God John Dalton (ca. 1803) elements by atomic weight JJ Thompson (ca. 1898) a 'plum pudding' model of the atom Ernest Rutherford (ca. 1910) a 'solar system' model of the atom - had major problems -- detailed later electrons should collapse (" crash ") through energy loss not explain light colors - atomic spectra not explain photoelectric effect Neils Bohr (1913) a new 'quantized' model which would solve those problems
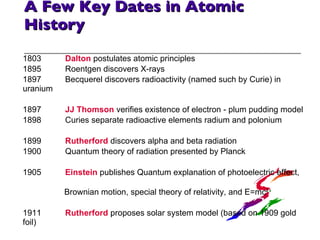
- 6. A Few Key Dates in Atomic History 1803 Dalton postulates atomic principles 1895 Roentgen discovers X-rays 1897 Becquerel discovers radioactivity (named such by Curie) in uranium 1897 JJ Thomson verifies existence of electron - plum pudding model 1898 Curies separate radioactive elements radium and polonium 1899 Rutherford discovers alpha and beta radiation 1900 Quantum theory of radiation presented by Planck 1905 Einstein publishes Quantum explanation of photoelectric effect, Brownian motion, special theory of relativity, and E=mc 2 1911 Rutherford proposes solar system model (based on 1909 gold foil) 1913 Bohr proposes quantum theory to modify Rutherford's model - ad hoc and ugly but explained problems
- 7. Evolution of the Atomic Model Democritus and early Greeks - sizes and shapes Dalton - ball with hooks next
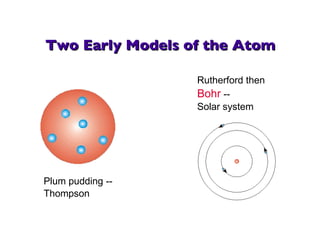
- 8. Two Early Models of the Atom Plum pudding -- Thompson Rutherford then Bohr -- Solar system
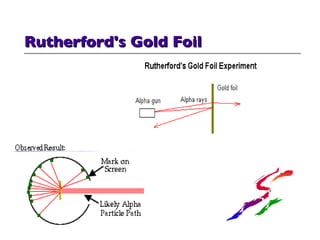
- 10. Early View of Atom - Rutherford's "Solar System" Model in 1911 Rutherford discovered nucleus in 'gold foil' bombardment experiment - some of the bullets ricocheted. Rutherford's Model looks good but will not work -- too many problems!
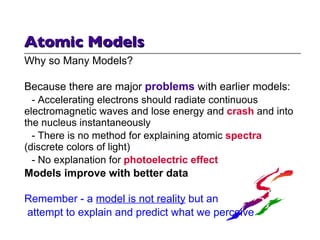
- 11. Atomic Models Why so Many Models? Because there are major problems with earlier models: - Accelerating electrons should radiate continuous electromagnetic waves and lose energy and crash and into the nucleus instantaneously - There is no method for explaining atomic spectra (discrete colors of light) - No explanation for photoelectric effect Models improve with better data Remember - a model is not reality but an attempt to explain and predict what we perceive
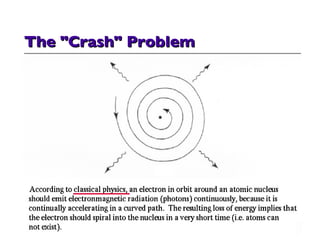
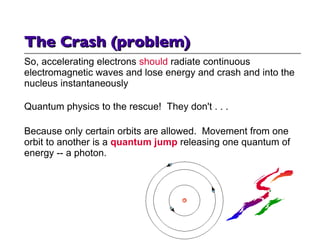
- 13. So, accelerating electrons should radiate continuous electromagnetic waves and lose energy and crash and into the nucleus instantaneously Quantum physics to the rescue! They don't . . . Because only certain orbits are allowed. Movement from one orbit to another is a quantum jump releasing one quantum of energy -- a photon. The Crash (problem)
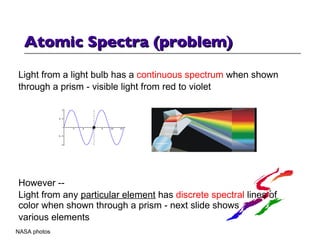
- 14. Atomic Spectra (problem) Light from a light bulb has a continuous spectrum when shown through a prism - visible light from red to violet However -- Light from any particular element has discrete spectral lines of color when shown through a prism - next slide shows various elements NASA photos
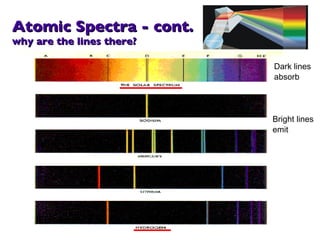
- 15. Atomic Spectra - cont. why are the lines there? Dark lines absorb Bright lines emit
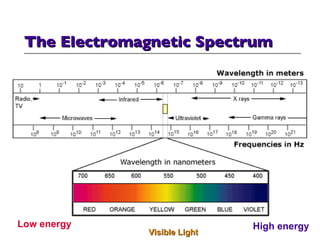
- 16. The Electromagnetic Spectrum Visible Light Low energy High energy
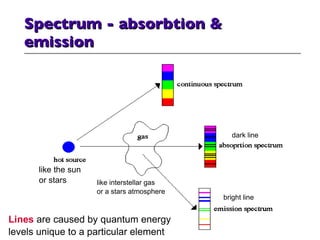
- 17. Spectrum - absorbtion & emission like the sun or stars like interstellar gas or a stars atmosphere dark line bright line Lines are caused by quantum energy levels unique to a particular element
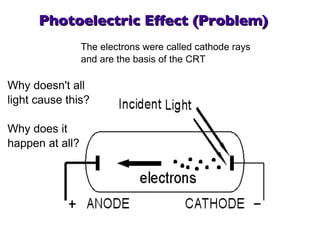
- 18. Photoelectric Effect (Problem) The electrons were called cathode rays and are the basis of the CRT Why doesn't all light cause this? Why does it happen at all?
- 19. The Photoelectric Effect Einstein to the rescue again: In 1905 he explained that light is not a wave with the energy spread out, but rather a concentrated particle-like 'bundle' of energy - photons A photon hits an atom and can knock an electron out For high energy photons, the ejection occurs at once For low energy photons, an electron will not be ejected counter intuitively, color (high or low energy) matters, intensity (brightness) does not We have now described the quantum and the quantum jump
- 20. Bohr's Atom
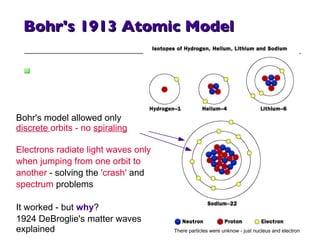
- 21. Bohr's 1913 Atomic Model Bohr's model allowed only discrete orbits - no spiraling Electrons radiate light waves only when jumping from one orbit to another - solving the 'crash' and spectrum problems It worked - but why ? 1924 DeBroglie's matter waves explained There particles were unknow - just nucleus and electron
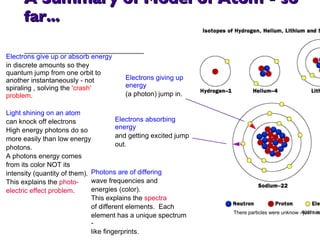
- 22. A Summary of Model of Atom - so far... Electrons absorbing energy and getting excited jump out. Photons are of differing wave frequencies and energies (color). This explains the spectra of different elements. Each element has a unique spectrum - like fingerprints. Electrons give up or absorb energy in discrete amounts so they quantum jump from one orbit to another instantaneously - not spiraling , solving the 'crash' problem . Light shining on an atom can knock off electrons High energy photons do so more easily than low energy photons. A photons energy comes from its color NOT its intensity (quantity of them). This explains the photo- electric effect problem . Electrons giving up energy (a photon) jump in. There particles were unknow - just nucleus and electron
- 23. 1919 Rutherford transmutes nitrogen to oxygen, verifies proton 1925 de Broglie proposes wave nature of electrons (why Bohr works) 1927 Heisenberg formulates uncertainty principle 1928 Dirac predicts positron, discovered in 1932 by Anderson 1930 Eddington explains where sun's energy originates (fusion) 1932 Chadwick discovers neutron 1933 Szilard proposes chain reaction by neutrons, 1934 patents atomic bomb, transfered to British navy in 1936 1934 Fermi transmutes elements to transuranics 1938 Hahn, Strassman split atom 1939 Meitner and Frisch explain Hahn's result (fission) 1940s 42-Manhattan, 45-Trinity tests, Japan bombed 1964 Gell-Mann suggests quarks as solution to particle explosion 1995 Top quark discovered (the last in the theory) A Few Key Dates in Atomic History - cont.
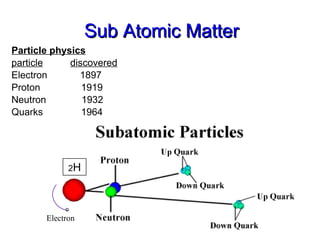
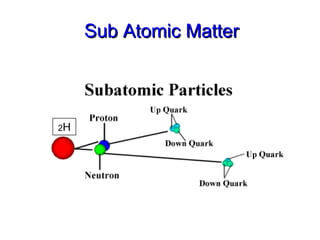
- 24. Sub Atomic Matter Particle physics particle discovered Electron 1897 Proton 1919 Neutron 1932 Quarks 1964 o Electron 2 H
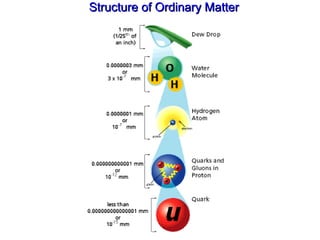
- 25. Structure of Ordinary Matter
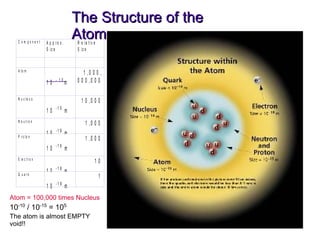
- 26. The Structure of the Atom Atom = 100,000 times Nucleus 10 -10 / 10 -15 = 10 5 The atom is almost EMPTY void!!
- 27. End of Atomic Development lots of good definitions and pictures follow for further discussion . . . END
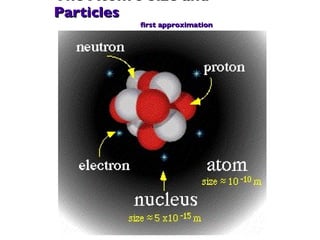
- 28. The Atom's Size and Particles first approximation
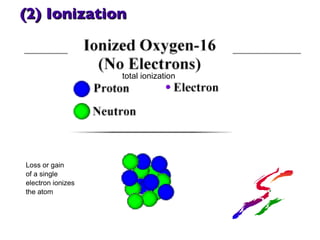
- 29. (2) Ionization Loss or gain of a single electron ionizes the atom total ionization
- 31. Simplification - Quarks and Leptons and the Fundamental Forces of Nature By the mid-twentieth century, there was a veritable zoo of particles and forces found by atom smashers A simplified scheme was eventually found that reduced all matter particles to just two types - quarks and leptons and all forces to just four types - gravity, electromagnetic, weak and color (strong) forces
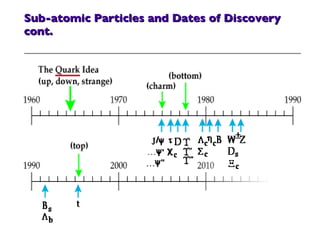
- 32. Sub-atomic Particles and Dates of Discovery cont.
- 33. Sub Atomic Matter 2 H
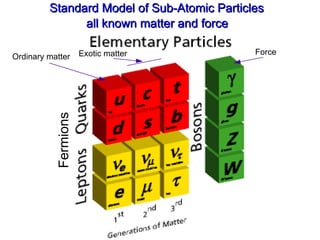
- 34. Standard Model of Sub-Atomic Particles all known matter and force Ordinary matter Force Exotic matter Fermions
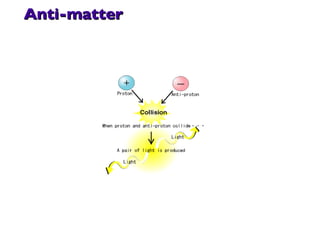
- 35. Anti-matter
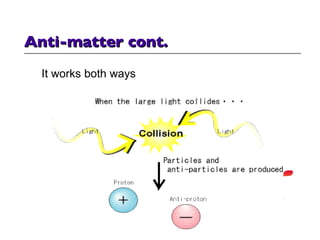
- 36. Anti-matter cont. It works both ways
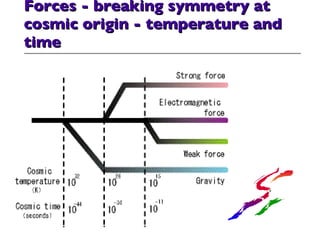
- 37. Forces - breaking symmetry at cosmic origin - temperature and time
- 39. Radioactivity - a spontaneous disintegration of atomic nuclei - nucleus emits alpha particles which are Helium nuclei beta particles which are electrons gamma particles which are electromagnetic rays and others - these react with other particles causing additional release of energy
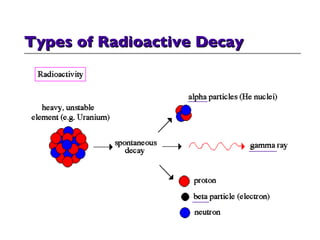
- 40. Types of Radioactive Decay
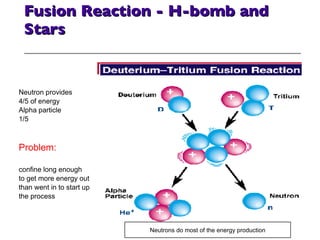
- 41. Fusion Reaction - H-bomb and Stars Neutron provides 4/5 of energy Alpha particle 1/5 Problem: confine long enough to get more energy out than went in to start up the process Neutrons do most of the energy production