Australian Dementia Network (ADNet)
- 2. Three Initiatives and Two Support Packages
- 3. ADNeT in a Nutshell ? Clinical Quality Registry (CQR) for newly diagnosed dementia or ¡°mild cognitive impairment¡± ? Memory Clinics ¨C expand, standardize and support ? Clinical Trial sites ¨C expand and support ? Longitudinal (n=4,000) and Trial ready (n=500) cohorts ¨C blood, MRI, cognition, amyloid and tau PET, +/- CSF every 2 years
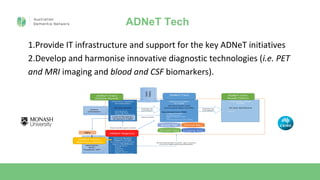
- 4. ADNeT Tech 1.Provide IT infrastructure and support for the key ADNeT initiatives 2.Develop and harmonise innovative diagnostic technologies (i.e. PET and MRI imaging and blood and CSF biomarkers).
- 5. The Australian Imaging Biomarkers and Lifestyle Study of Ageing . Commenced late 2006
- 6. 0m 18m 36m 54m 72m 90m 108m 126m CN 1476 1034 776 684 523 419 376 219 MCI 394 174 107 79 59 34 28 19 AD 427 286 219 127 68 44 30 6 Other 17 7 10 12 8 6 3 2 Total 2314 1501 1112 902 658 503 437 246 Cumulative Withdrawn 468 681 838 986 1066 1129 1153 % Follow up complete 81% 68% 61% 49% 40% 37% 21% AIBL cohort : Number of assessments
- 7. 3,500 researchers granted access to AIBL data via www.adni.loni.usc.edu Includes access granted to 112 companies e.g.: Abbott Labs, Abiant, ADM diagnostics, Astra Zeneca, Avid, BioClinica, Biogen Idec, Bristol-Myers Squibb, Cogstate Cytokinetics, Eisai, Elan, Eli Lilly, GE Health Care, General Resonance, Genetech, Imorphics, Iris Biotechnologies, Janssen, Johnson Johnson, M and M Scientific, Merck & Co, Mimvista, Pentara Corp, Pfizer, Philips, Predixion software, Rancho Biosciences, Servier, Siemens, Soft team solutions, UCB, United Biosource Corp. AIBL has also received 250 Expressions of Interest for access to more detailed data. Bangladesh China Hong Kong India Indonesia Japan Kyrgyzstan Malaysia Pakistan Philippines Singapore South Korea Taiwan Thailand Australia New Zealand Iran Iraq Israel Saudi Arabia Turkey Canada Cuba Martinique Mexico Puerto Rico USA Argentina Bolivia Brazil Chile Colombia Ecuador Guyana Netherlands Antilles Peru Uruguay Austria Belgium Bulgaria Cyprus Denmark Estonia Finland France Algeria Egypt Ghana Morocco Nigeria Tunisia Germany Greece Hungary Ireland Italy Lithuania Luxembourg Macedonia Netherlands Norway Poland Portugal Romania Russia Serbia Slovakia Slovenia Spain Sweden Switzerland Ukraine UK