Balencio por redox
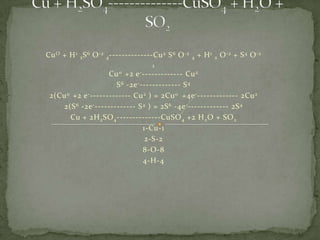
- 1. CuO + H1 2S6 O-2 4--------------Cu2 S6 O-2 4 + H1 2 O-2 + S4 O-2 2 Cu0 +2 e-------------- Cu2 S6 -2e-------------- S4 2(Cu0 +2 e-------------- Cu2 ) = 2Cu0 +4e-------------- 2Cu2 2(S6 -2e-------------- S4 ) = 2S6 -4e-------------- 2S4 Cu + 2H2SO4--------------CuSO4 +2 H2O + SO2 1-Cu-1 2-S-2 8-O-8 4-H-4
- 2. ï Mg 1 ï Cu = 63 x1 ï 63 g/m ï Mg 2 ï H= 1 x 4 = 4 ï S= 32 x 2 = 64 ï O=16 x 8 = 128 ï 196 m/g ï Mg3 ï Cu= 63 x1 =63 ï S= 32 x1= 32 ï O= 16 x 4= 64 ï =159 m/g ï Mg4 ï H= 1x4= 4 ï O= 16 x 2= 32 ï =36 m/g Mg 5 S =32 x 1 = 32 O= 16 x 2 = 32 =64 m/g
- 3. Fe3 2 O-2 3 + C2 O-2 ------------ Fe0 + C4 O-2 2 Fe3 -3e- ------------ Fe0 C2+2e ------------ C4 2(Fe3 -3e- ------------ Fe0 ) = 2Fe3 -6e- ------------ 2Fe0 3(C2+2e ------------ C4 ) = 3C2+6e ------------ 3C4 Fe2 O3 + 3CO ------------ 2Fe +3CO2 2-Fe-2 6-O-6 3-C-3
- 4. ï Mg 1 ï Fe= 56 x 2 =112 ï O = 16 x 3 =48 ï = 160 m/g ï Mg 2 ï C = 12 x 3 = 36 ï O= 16 x 3 =48 ï =84 m/g ï Mg 3 ï Fe= 56 x 2= 112 ï =122 m/g ï Mg 4 ï C =12 x 3 = 36 ï O=16 x 6 = 96 ï =132 m/g