Barbiturates
- 1. By; AMJAD ANWAR Department of Pharmacy, UOM
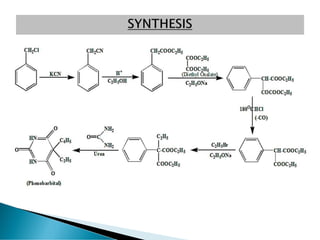
- 2. ? All Barbiturates are the derivatives of Barbituric acid, formed by the reaction of Malonic acid with Urea. ? Barbituric acid was first synthesized on Dec 6, 1864 by German researcher Adolf Von Baeyeri, by the reaction of Malonic acid with Urea.
- 3. GENERAL STRUCTURE ? Note: ? In Thiopental and Thiamylal (Ultra Short acting), X = S while in rest of all X = O
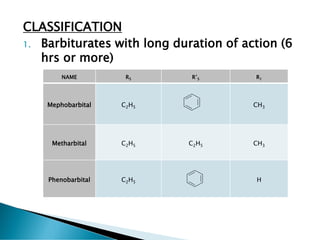
- 4. CLASSIFICATION 1. Barbiturates with long duration of action (6 hrs or more) NAME R5 RĄä5 R1 Mephobarbital C2H5 CH3 Metharbital C2H5 C2H5 CH3 Phenobarbital C2H5 H
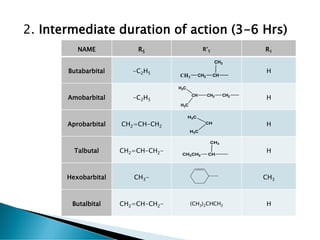
- 5. 2. Intermediate duration of action (3-6 Hrs) NAME R5 RĄä5 R1 Butabarbital -C2H5 H Amobarbital -C2H5 H Aprobarbital CH2=CH-CH2 H Talbutal CH2=CH-CH2- H Hexobarbital CH3- CH3 Butalbital CH2=CH-CH2- (CH3)2CHCH2 H CH3 CH2 CH CH3 CH H3C H3C CH2 CH2 CH H3C H3C CH3CH2 CH CH3
- 6. 3. Short duration of action (less than 3 Hrs) NAME R5 RĄä5 R1 Pentobarbital -C2H5 H Secobarbital CH2=CH-CH2- H CH3 CH2 CH2 CH CH3 CH3 CH2 CH2 CH CH3
- 7. 4. Ultra short acting NAME R5 RĄä5 R1 Thiopenton or Thiopental -C2H5 H Thiamylal CH2=CH-CH2- HCH3 CH2 CH2 CH CH3 CH3 CH2 CH2 CH CH3
- 8. Chemical Name ? 5-Ethyl, 5-Phenyl 2,4,6 pyrimidinetrione Description: ? It occurs as white, odorless, glistering small crystals or as a white crystalline powder, which may exhibit polymorphism ? M.P is about 176 oC ? Its PKa is 7.6 Solubility: ? 1gm of it is soluble in; ? 1000ml of water ? 10ml alcohol ? 40ml of chloroform ? & 15ml of ether.
- 10. Chemical name ? 5-(1-cyclohexene-1-yl) 1,5-dimethyl barbituric acid Description/physical properties: ? See Remington or google
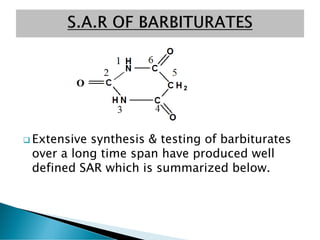
- 12. ? Extensive synthesis & testing of barbiturates over a long time span have produced well defined SAR which is summarized below.
- 13. 1) Both H atoms at position # 5 should be replaced, if a single H is present at position # 5, then it will give rise to phenomena of tantomerism, the resultant compound will be highly acidic. Tri-OH pyrimidine, having Pka = 4 is largely in ionic form (anionic form) at physiological PH with little unionized lipid soluble portion to cross BBB. 2) Beginning with the lower alkyl group, there is an increase of onset of action. % increasing of hydrocarbon contents upto 7-9 carbon atom substituted at position # 5, there is an increase in onset of action & decrease in duration of action, so for optimal activity the sum of carbon atoms at position # 5 having substituents would be 7-9. ? Increase in onset of action may be due to enhanced lipophilicity & ability of drug atom to penetrate brain & ? Decrease in duration of action may be due to its ability to penetrate liver microsomes.
- 14. 3) N-methylation at position # 3 shortens onset time & duration of action as compared with the weaker acid (barbiturates without N- methyl group i.e phenobarbitone with Pka 7.6). The weaker acid (N-methylated derivative) is an unionized lipid soluble form which can readily cross CNS.
- 15. 4) Introduction of halogen atom to 5 alkyl substituents will enhance the activity 5) Introduction of polar groups like OH, COOH, NH2, SO3H, to 5-alkyl substituent gives derivatives which are inactive. 6) Replacement of carbonyl oxygen at Carbon # 2 will results in Thiobarbital with quick onset & shorter duration of action i.e Thiopental & Thiamylal. ? Thiobarbiturates differs a little from barbiturates in acid strength values, the Pka value of Thiopental is 7.4 as compared with carbonyl barbiturates with Pka 7 which may be due to lipophilic character of sulphur atom.