battery-131208120748-phpapp02 (1).pdf
- 1. Battery
- 2. collection of one or more electrochemical cells in which stored chemical energy is converted into electrical energy
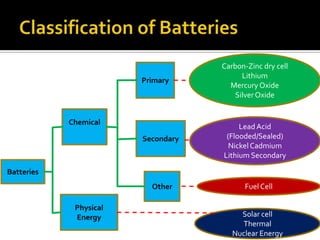
- 3. Batteries Other Secondary Primary Physical Energy Chemical Carbon-Zinc dry cell Lithium Mercury Oxide SilverOxide Fuel Cell Solar cell Thermal Nuclear Energy Lead Acid (Flooded/Sealed) NickelCadmium Lithium Secondary
- 4. Deep-cycle Designed for maximum energy storage capacity and high cycle count (long life), and are rated in Amp/Hours. This is achieved by installing thick lead plates with limited surface area. Typical applications are boats, Uninterruptible Power Supplies (UPS) Engine Starting Starter batteries are made for maximum power output, usually rated in CCA (Cold-Cranking amps). The battery manufacturer obtains this by adding multiple Ī░lead platesĪ▒ to obtain larger surface area for maximum conductivity. Typical applications are vehicles & motorcycles
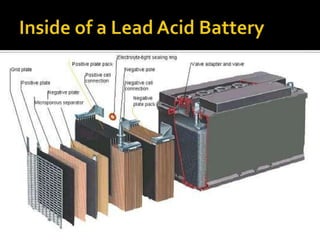
- 5. Lead-acid batteries are commonly made of five basic components ? A resilient plastic container ? Positive and negative internal plates made of lead ? Plate separators made of porous synthetic material ? Electrolyte - 35% sulfuric acid and 65% water ? Battery Terminals
- 6. Lead-Acid Batteries come in several different configurations ? Flooded Lead-acid ©C Available in Deep cycle or Engine starting as sealed or open variety ? Sealed Lead-acid - The liquid electrolyte is gelled into moistened lead plate-separators, which allow the case to be sealed. Safety valves allow venting during charge, discharge and atmospheric pressure changes. ? Absorbed Glass Mat Batteries (AGM) - sealed lead-acid that uses absorbed glass mats between the plates. It is sealed, maintenance-free and the plates are rigidly mounted to withstand extensive shock and vibration
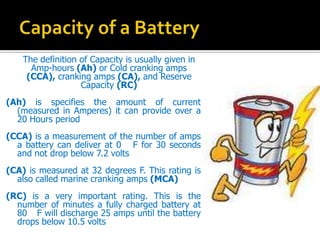
- 7. The definition of Capacity is usually given in Amp-hours (Ah) or Cold cranking amps (CCA), cranking amps (CA), and Reserve Capacity (RC) (Ah) is specifies the amount of current (measured in Amperes) it can provide over a 20 Hours period (CCA) is a measurement of the number of amps a battery can deliver at 0 F for 30 seconds and not drop below 7.2 volts (CA) is measured at 32 degrees F. This rating is also called marine cranking amps (MCA) (RC) is a very important rating. This is the number of minutes a fully charged battery at 80 F will discharge 25 amps until the battery drops below 10.5 volts
- 10. Parameters for Recharging ? Charging Current - All batteries have a Ī░maximum currentĪ▒ at which they can be safely charged ? Charging Voltage - Applying a voltage across its positive & negative terminals that is higher than the voltage it already has across them ? Charging Time - The charge time of a sealed lead- acid battery is 12-16 hours (up to 36 hours for larger capacity batteries)
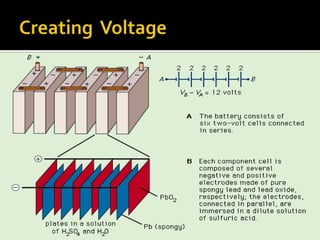
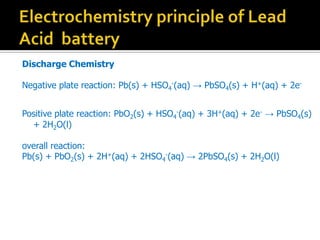
- 11. Discharge Chemistry Negative plate reaction: Pb(s) + HSO4 -(aq) Ī· PbSO4(s) + H+(aq) + 2e- Positive plate reaction: PbO2(s) + HSO4 -(aq) + 3H+(aq) + 2e- Ī· PbSO4(s) + 2H2O(l) overall reaction: Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4 -(aq) Ī· 2PbSO4(s) + 2H2O(l)
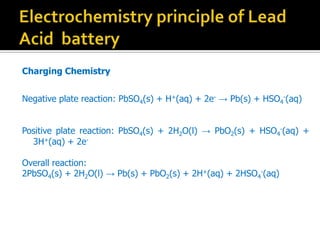
- 12. Charging Chemistry Negative plate reaction: PbSO4(s) + H+(aq) + 2e- Ī· Pb(s) + HSO4 -(aq) Positive plate reaction: PbSO4(s) + 2H2O(l) Ī· PbO2(s) + HSO4 -(aq) + 3H+(aq) + 2e- Overall reaction: 2PbSO4(s) + 2H2O(l) Ī· Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4 -(aq)
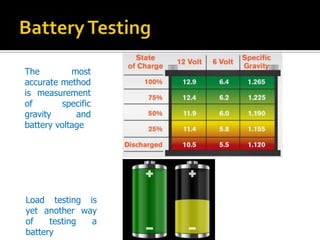
- 13. The most accurate method is measurement of specific gravity and battery voltage Load testing is yet another way of testing a battery
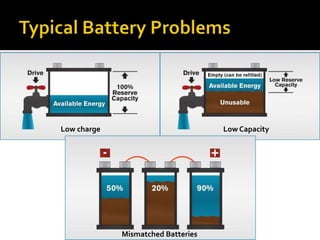
- 15. Low charge Low Capacity Mismatched Batteries
- 16. Conversion Efficiency This denotes how well it converts an electrical charge into chemical energy and back again. The higher this factor, the less energy is converted into heat and the faster a battery can be charged without overheating. The lower the internal resistance of a battery, the better its conversion efficiency. Sulfation Sulfation of lead-acid batteries starts when the electrolyteĪ»s specific gravity falls below 1.225. It results in a salt-like substance forming on the battery plate surface and it can harden on the battery plates if left long enough, reducing and eventually blocking chemical reaction between the lead plate and the electrolyte. Equalization is the solution for this problem.
- 17. Gassing Batteries start to gas when you attempt to charge them faster than they can absorb the energy. The excess energy is turned into heat, which then causes the electrolyte to boil and evaporate. is the suitable method for reduce this is good ventilated area. Self-Discharge The self-discharge rate is a measure of how much batteries discharge on their own. The self-discharge rate is governed by the construction of the battery and the properties of the components used inside the cell (alloy of the lead, sulfuric concentrations of the electrolyte, etc.).
- 18. The optimum operating temperature for the lead-acid battery is 25 C (77 F). As a guideline, every 8 C (15 F) rise in temperature will cut the battery life in half. A VRLA, which would last for 10 years at 25 C (77 F), will only be good for 5 years if operated at 33 C (95 F). Theoretically the same battery would last a little more than one year at a desert temperature of 42 C (107 F)
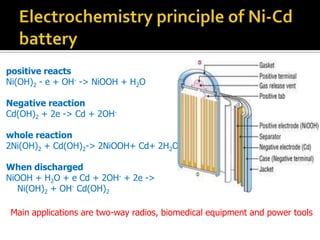
- 20. positive reacts Ni(OH)2 - e + OH- -> NiOOH + H2O Negative reaction Cd(OH)2 + 2e -> Cd + 2OH- whole reaction 2Ni(OH)2 + Cd(OH)2-> 2NiOOH+ Cd+ 2H2O When discharged NiOOH + H2O + e Cd + 2OH- + 2e -> Ni(OH)2 + OH- Cd(OH)2 Main applications are two-way radios, biomedical equipment and power tools
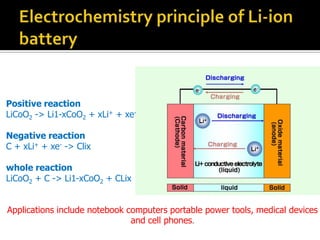
- 21. Positive reaction LiCoO2 -> Li1-xCoO2 + xLi+ + xe- Negative reaction C + xLi+ + xe- -> Clix whole reaction LiCoO2 + C -> Li1-xCoO2 + CLix Applications include notebook computers portable power tools, medical devices and cell phones.
- 22. Ensure proper maintenance of engine starting batteries due to the extreme importance of getting a ship under way in any circumstances ? Attention should be paid to the electrolyte level and specific gravity for vented batteries ? A boost charge shall be given if the specific gravity of the battery cells meet the conditions stipulated by manufacturer ? Ensure that the battery is not being overcharged ? Keep engine starting batteries clean, dry and free of seawater ? Period of inactivity for the ship of a week or more, give the battery a normal charge
- 23. ? Inspected for height of electrolyte once each week ? The electrolyte level shall never be allowed to fall below the top of the separators ? Add pure distilled water at any time to replace that which has evaporated ? Add water just before the battery is placed on charge, as the water remains on top of the electrolyte until mixed with it by charging ? After adding water, replace and tighten the vent plugs ? Remove all water or electrolyte spilled during watering and make sure that the tops and sides of the cells are clean and dry
- 24. ? Ensure that distilled water that is to be used for watering batteries and mixing electrolyte does not contain impurities ? Use only premixed electrolyte when replacing spilled electrolyte ? Fully charged specific gravity between the limits of 1.220 and 1.210 specific gravity at 27 C (80 F) ? The specific gravity of a cell that has fallen below 1.210 shall not be increased by the addition of acid untill it has been definitely ascertained by test that the low-gravity condition is not due to sulfation ? The addition of acid to increase the specific gravity of a sulfated cell will aggravate the existing condition
- 25. ? The specific gravity of cells which exceed 1.220 shall be cut by the removal of an appropriate amount of electrolyte and the addition of distilled water ? Sulfuric acid of a specific gravity greater than 1.350 shall not be added to a battery
- 26. ? Personnel handling or mixing electrolyte shall wear proper protective items ? If concentrated acid or electrolyte come in contact with the skin, immediately wash the affected with freshwater ? As soon as possible get the medical assistance ? During electrolyte mixing the acid must be poured into the water and not the water poured into the acid ? The acid must be added slowly and cautiously to the water to prevent excessive heating and splashing ? The solution should be continually stirred by a glass rod while the acid is being poured into the water to prevent the heavier acid from flowing to the bottom of the vessel
- 27. ? To prepare electrolyte, lead or rubber vessels and stirring rods are necessary ? Only pure distilled water shall be used ? Every effort must be made to keep impurities from the electrolyte while mixing, since they shorten battery life ? Extreme care must be taken to ensure that acid container (carboys) are absolutely airtight ? The addition of even a small quantity of water to a carboy of strong sulfuric acid may cause an explosion due to the sudden evolution of heat