Benzoin condensation
- 1. BENZOIN CONDENSATION PRESENTED BY: MR K V NANDA KUMAR ASSOCIATE PROFESSOR KRISHNA TEJA PHARMACY COLLEGE, TIRUPATHI-517506 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 1
- 2. CONTENTS 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 2 History General principle Requirements for the reaction Reaction mechanism Applications
- 3. HISTORY 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 3 The Benzoin condensation is a condensation reaction between two aromatic aldehydes, especially benzaldehyde that is catalyzed by a cyanide. The reaction product is an aromatic acyloin with benzoin as the parent compound. The history of this reaction goes back to 1832 when it was reported by German scientists Justus von Liebig and Friedrich WÃķhler.
- 4. GENERAL PRINCIPLE âĒ The benzoin condensation is the condensation between two molecules of benzaldehyde to form benzoin in the presence of a cyanide catalyst (e.g., NaCN and KCN) or thiamine (vitamin B). âĒ The structure of benzoin is that of a ketone. âĒ It consists of acetophenone bearing hydroxy and phenyl substituents at the alpha-position (Îąâhydroxyl ketone). âĒ Its IUPAC name is 2-hydroxy-1,2-diphenylethanone with a molecular mass of 212.24 g/mol. âĒ A cyanide ion usually catalyzes benzoin condensation. 4 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR
- 5. REQUIREMENTS OF THE REACTION âĒ Two molecules of benzaldehyde âĒ A cyanide ion usually catalyzes benzoin condensation. âĒ Cyanide is used because it is a good nucleophile, can stabilize the intermediate ion, and is an excellent leaving group. 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 5
- 6. MECHANISM OF THE REACTION 6/19/2020 6 PHARM D YEAR I POC - MR K V NANDA KUMAR âĒ STEP 1: ïąThe standard method for performing the benzoin condensation begins with benzaldehyde treated with a catalytic amount of sodium cyanide in the presence of a base. ïąThe cyanide ion forms a stable cyanohydrin intermediate
- 7. MECHANISM OF THE REACTION 6/19/2020 7 PHARM D YEAR I POC - MR K V NANDA KUMAR âĒ STEP 2: ïąThe second step is the condensation reaction that occurs between the cyanohydrin and the benzaldehyde.
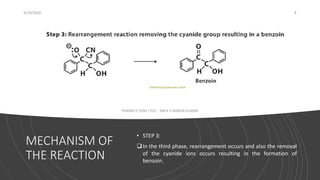
- 8. MECHANISM OF THE REACTION 6/19/2020 8 PHARM D YEAR I POC - MR K V NANDA KUMAR âĒ STEP 3: ïąIn the third phase, rearrangement occurs and also the removal of the cyanide ions occurs resulting in the formation of benzoin.
- 9. USES AND APPLICATIONS OF BENZOIN CONDENSATION 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 9 The benzoin condensation reactions find uses in various organic synthesis and reactions. The product benzoin is made use in hardening of different polymers by making use of microemulsion. The reaction is helpful in the synthesis of heterocyclic compounds and also extends to the aliphatic form of aldehydes. These reactions also find its applications in the poly chemistry, for the production of polymers as well in the condensation of new monomers.
- 10. QUESTIONS âĒ What is benzoin condensation? âĒ Write the general principle involved in benzoin condensation. âĒ Explain the mechanism involved in benzoin condensation? âĒ What are the important applications of benzoin condensation? 6/19/2020 PHARM D YEAR I POC - MR K V NANDA KUMAR 10
- 11. 11 Thank you Any questions