Best presentation of uv
- 2. Contents 2
- 3. INTRODUCTION ? UV visible spectroscopy is also known as electronic spectroscopy in which, the amount of light absorbed at each wavelength of UV and visible regions of electromagnetic spectrum is measured. ? This absorption of electromagnetic radiations by the molecules leads to molecular excitations. 3
- 4. Two ranges of electromagnetic radiations in UV visible spectroscopy ULTRA VIOLET ULTRAVIOLET REGION(10-380)n VISIBLE VISIBLE REGION (380-780)nm 4
- 5. UV region There are two regions in UV (1) Vacuum ultra violet region This region comprises below 200 nm in this region oxygen absorbs strongly , but range of instrument can be extended down to 150 nm , by flushing nitrogen instead of oxygen.If the instrument is evacuated,it is possible to study the whole UV region even below 200nm.the region below 200nm is therefore usually known as vacuum ultraviolet region. (2)Ordinary ultraviolet region This region comprises 200-400nm because atmosphere is transparent in this region. 5
- 6. PRINCIPLE As the result of absorption of electromagnetic radiation molecular transitions occur. These transition occur between the electronic energy levels.As molecule absorbs energy , an electron is promoted from occupied orbital to an unoccupied orbital of greater potential energy.Generally the most probable transition is from(HOMO) to(LUMO). 6
- 7. 7
- 8. FOUR TYPES OF ELECTRONIC TRANSITIONS 1 2 ĶŌĄúĶŌ* for an ordinary carbon-carbon bond. (125-150)nm ĶÐĄúĶÐ* for an isolated double bond. (160190)nm 3 nĄúĶÐ* In carbonyl compounds.Aldehydes And ketones(275-295)nm 4 nĄúĶŌ* in oxygen,nitrogen ,sulphur and Halogen compounds.(150-250)nm 8
- 9. Electronic energy levels and transitions 9
- 10. INSTRUMENTATION Two types of instruments can be used (1)Single beam spectrophotometer A single-beam instrument uses only single beam of radiation through a single cell.The refrence cell is used to set the absorbance scale at zero for the wavelength to be studied.It is then replaced by sample cell to determine the absorbance of the sample at that wavelength. 10
- 12. (2)Double beam instrument A double beam instrument divides the radiation into two beams of equal intensity which are passed through two separate cells. one of the two cells contain the sample solution, while other, called the reference cell, contains either the pure solvent or a blank solution.Since the absorption by the sample is automatically corrected for absorption occurring in the solvent, the readout from the instrument is the difference between amounts of the radiations absorbed in the two cells. 12
- 13. Working of double beam instrument 13
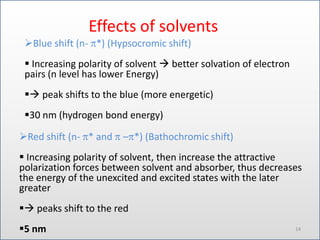
- 14. Effects of solvents ?Blue shift (n- *) (Hypsocromic shift) ? Increasing polarity of solvent ? better solvation of electron pairs (n level has lower Energy) ?? peak shifts to the blue (more energetic) ?30 nm (hydrogen bond energy) ?Red shift (n- * and ĻC *) (Bathochromic shift) ? Increasing polarity of solvent, then increase the attractive polarization forces between solvent and absorber, thus decreases the energy of the unexcited and excited states with the later greater ?? peaks shift to the red ?5 nm 14
- 15. 15
- 16. Wavelength absorbed by functional groups 16
- 17. EXAMPLE 17
- 18. Example of a Method to Determine the Absorption Spectra of an Organic Compound WoodwardĄŊs Rules For Conjugated Carbonyl Compounds Aldehyde: Extended conjugation: Homodyne component: 208 nm 30 nm 39 nm a-Alkyl groups or ring residues: 10 nm d-Alkyl groups or ring residues: 18 nm Calculated: 304 nm 18
- 19. APPLICATIONS ? Structure determination As different chromosphores absorbs at different wavelength, it is some times possible to identify the absorbing chromophore in an unknown compound by comparing the wavelength of its maximum absorption with the absorption characteristics of known compounds. 19
- 20. ? Analytical application Since UV/visible absorption bands are usually intense, small quantities of an observing substance can be easily detected . ? Stereo chemical studies The absorption by a conjugated system is due to the ĶÐĄúĶÐ* transition. In the conjugated system the conjugation is decreased from preventing coplanarity, which affects the absorption spectrum.The UV/visible, therefore, provides useful tool for studying steriochemical details in certain molecules. 20