Calibration
Download as PPTX, PDF4 likes2,568 views
Calibration as important aspect of pharma industry Purpose, frequency of calibration and methods to find out calibration frequency of equipment
1 of 11
Download to read offline






Recommended
Calibration



CalibrationVivek Jain
Ěý
Validation ensures that processes and methods consistently produce results within specifications. Qualification proves equipment works correctly and leads to expected results through testing. Calibration compares instrument measurements to standards to ensure accuracy. A Validation Master Plan summarizes an organization's validation strategy and ensures resources are available for validation projects. It discusses validation activities across the organization.Calibration and Qualification



Calibration and QualificationPriyanka Kandhare
Ěý
This document discusses calibration and qualification. It begins by defining calibration as comparing a measurement system or device of unknown accuracy to a standard of known accuracy. The objectives of calibration are to check accuracy, determine traceability, and provide confidence that results meet specifications. Calibration is important to detect drift over time and ensure measurements remain reliable. Qualification establishes that equipment is suitable for its intended use and performs properly. It includes design qualification to define specifications, installation qualification to confirm proper installation, operational qualification to demonstrate functional specifications are met, and performance qualification to establish consistent performance.Calibration



CalibrationPoornima institute of engineering and technology
Ěý
The document discusses calibration, including defining calibration as checking the accuracy of measuring instruments against a standard. It describes various calibration laboratories and standards in India such as NPL, ERTL, and ETDC. It explains the importance, purpose, and types of calibration, as well as requirements for calibration management systems and common instrument calibrations.Qualification of instrumets



Qualification of instrumetsChowdaryPavani
Ěý
This document discusses the qualification and calibration of analytical instruments and glassware. It describes the components of analytical data quality including qualification, calibration protocols, and the need for calibration. It then provides details on calibrating specific instruments like electronic balances, pH meters, UV-visible spectrophotometers, FTIR, GC, HPLC, and HPTLC. It also covers calibrating various glassware items like volumetric flasks, pipettes, measuring cylinders, and beakers. The calibration procedures and acceptance criteria are outlined for ensuring the accuracy of measurements from analytical equipment.CALIBRATION OF pH METER 



CALIBRATION OF pH METER CT University Ludhiana
Ěý
this document includes calibration, procedure of calibration, calibration, validation,
calibration of ph meterQualification of UV spectrophotometer 



Qualification of UV spectrophotometer Raghavendra institute of pharmaceutical education and research .
Ěý
in this slide contains Qualification of UV spectrophotometer
presented by: G.CHIRANJEEVI (Department of pharmaceutical analysis).RIPER, anantapurEquipment calibration PPT by Shravan Kumar



Equipment calibration PPT by Shravan Kumarshravan dubey
Ěý
This presentation is for Pharmaceuticals Equipments which are used in the manufacturing of APIs. Calibration procedure for Equipmentscalibration-and-validation



calibration-and-validationSUJITHA MARY
Ěý
This document discusses concepts related to validation in the pharmaceutical industry. It begins by defining validation as proving that a process consistently produces the expected results. There are three phases of validation: pre-validation to establish the design; process qualification to evaluate the design; and continued process verification. Validation is important for quality assurance and regulatory compliance. The key types of validation discussed are process validation, cleaning validation, and analytical method validation.Calibration



CalibrationPradeep Kumar
Ěý
Calibration establishes the relationship between instrument measurements and known standard values through a series of steps. Key aspects of calibration include identifying instruments and sources, following calibration procedures, documenting results, accounting for sources of error, and ensuring traceability to national standards. Calibration procedures vary based on instrument type, but generally involve evaluating instrument performance, establishing calibration curves using certified reference materials at multiple concentration levels, and quantifying samples based on the calibration curves.PPT ON GOOD LABORATORY PRACTICES (GLP)



PPT ON GOOD LABORATORY PRACTICES (GLP)GOVIND YADAV
Ěý
The document discusses Good Laboratory Practice (GLP) guidelines. It notes that in the 1970s, the FDA discovered many cases of poor laboratory practices and fraudulent activities in the US. This led to the establishment of GLP principles to ensure reliable and high-quality non-clinical safety studies. GLP guidelines cover facilities, equipment, standard operating procedures, personnel training, record-keeping, and quality assurance programs. The goal of GLP is to ensure that experimental data accurately reflect the results and that studies are conducted in accordance with the principles of good laboratory practice.Analytical method validation as per ich and usp 



Analytical method validation as per ich and usp shreyas B R
Ěý
Analytical method validation is a process of documenting/ proving that an analytical method provides analytical data acceptable for the intended use.After the development of an analytical procedure, it is must important to assure that the procedure will consistently produce the intended a precise result with high degree of accuracy. The method should give a specific result that may not be affected by external matters. This creates a requirement to validate the analytical procedures. The validation procedures consists of some characteristics parameters that makes the method acceptable with addition of statistical tools.Qualification of analytical instruments



Qualification of analytical instrumentsFaris ameen
Ěý
This document provides guidelines for qualifying analytical instruments including electronic balances, pH meters, and UV-Visible spectrophotometers. It discusses the various levels of qualification including: Level I which involves selecting instruments and suppliers; Level II which involves installation and releasing instruments for use; Level III which involves periodic checks; and Level IV which involves in-use checks. Specific guidelines are provided for qualifying balances, pH meters, and UV-Visible spectrophotometers, including recommended tolerance limits for various parameters, calibration procedures, and qualification frequencies.Qualification of UV VISIBLE SPECTROPHOTOMETER



Qualification of UV VISIBLE SPECTROPHOTOMETERDr.K.Venkateswara raju
Ěý
This document discusses the qualification of UV-visible spectrophotometry. It begins by defining qualification as an act or process to ensure something complies with conditions, standards, or requirements. There are four types of qualification: design, installation, operational, and performance. UV-visible spectroscopy is concerned with the ultraviolet and visible regions ranging from 200-780 nm. The document outlines parameters for acceptance procedures and performance qualification of a UV-visible spectrophotometer, including wavelength accuracy, stray light, resolution power, noise, baseline flatness, stability, photometric accuracy, and linearity.Quality audit



Quality auditutkarsha shivsharan
Ěý
Quality audits involve the systematic and independent examination of quality activities and processes to determine compliance with established standards. They can be internal or external. The presented document discusses the definition, purpose, types, procedures, and important aspects of quality audits for pharmaceutical companies. It provides an overview of audit planning, conducting audits, common difficulties found, and essential features of audit reports. The goal is to evaluate procedures, records, and operations to ensure they meet quality goals and identify any non-compliances or deficiencies.calibration of analytical instruments 



calibration of analytical instruments Lavanya Pinneboina
Ěý
Calibration of analytical instruments is important to ensure they are accurate and precise. It involves comparing an instrument's measurements to a reference standard to determine any adjustments needed. Regular calibration helps verify that instruments are suitable for their intended purposes in pharmaceutical analysis. It is necessary to comply with quality standards and regulations. The calibration process involves using traceable standards and documented procedures to evaluate instruments and certify their performance is within specified tolerance limits. Maintaining calibration records provides a history of each instrument's accuracy over time.Validation master plan



Validation master planDr. Amsavel A
Ěý
The Validation Master Plan (VMP) outlines the company's approach to validation. It defines responsibilities, schedules, and documentation requirements for qualification of facilities, equipment, and processes. The VMP ensures management understands validation needs and the validation team understands their tasks. Key elements include qualification of equipment and facilities, process validation, cleaning validation, change control procedures, and periodic revalidation. Qualification includes design, installation, operational, and performance qualification to confirm equipment and facilities operate as intended. Process validation demonstrates manufacturing processes consistently produce products meeting specifications. The VMP helps regulatory inspectors evaluate the company's validation program.Qualification of GC & FTIR



Qualification of GC & FTIRMehulJain143
Ěý
The document provides details on qualification and validation procedures for gas chromatography (GC) and Fourier transform infrared spectroscopy (FTIR) systems used in pharmaceutical analysis. It describes calibration tests for both instruments, including tests to check wave number precision and reproducibility, linearity, and temperature accuracy and stability. Validation tests are also outlined, such as resolution checks and tests to evaluate performance against acceptance criteria over time as specified in guidance documents. The document provides a thorough overview of qualification processes for critical analytical instruments.Analytical method validation



Analytical method validationDevipriya Viswambharan
Ěý
This document discusses analytical method validation. It defines analytical method validation as providing assurance that an analytical method can consistently and accurately determine the presence or quantity of attributes. The objectives of validation are to obtain consistent, reliable and accurate data. Key parameters that are assessed in validation include specificity, accuracy, precision, linearity, range, limits of detection and quantification, ruggedness and robustness. The validation process involves planning, testing method performance characteristics, selecting validation acceptance criteria, and documenting results in a validation report. Validation is important for analytical methods used in pharmaceutical analysis.Basics of Impurity Profiling



Basics of Impurity ProfilingPankaj Soni
Ěý
describes what are impurities, sources of impurity, applications of impurity profiling and how to do impurity profiling.SITE ACCEPTANCE TEST



SITE ACCEPTANCE TESTRaghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains SITE ACCEPTANCE TEST IN QUALIFICATION.
Presented by: K. SANDHYA RANI (Department of pharmaceutical analysis ).RIPER, anantapurAnalytical method validation



Analytical method validationGaurav Kr
Ěý
This document discusses guidelines for analytical method validation. It outlines types of analytical methods that require validation including chromatographic, spectroscopic, and dissolution methods. Key analytical performance characteristics used in validation are described such as specificity, linearity, range, accuracy, precision, detection/quantitation limits, robustness, and system suitability testing. The document provides details on determining these characteristics and validating methods. It also addresses revalidation and references for further information.Validation Protocol



Validation ProtocolSagar K Savale
Ěý
ValidationĚýis a documented program that provides high degree of assurance that a specific process, method or system consistently produces a result meeting pre-determined acceptance criteria.Residual solvent 



Residual solvent Saptarshi Das
Ěý
This document summarizes the determination of residual solvents in pharmaceuticals by gas chromatography according to ICH guidelines. Residual solvents are organic chemicals used in manufacturing that are not fully removed and may pose health risks. They are classified into Class 1, 2, or 3 based on toxicity, with Class 1 solvents prohibited. Gas chromatography with headspace injection and flame ionization detection is the primary analytical method. It involves using capillary columns, temperature programs, and identifies solvents by comparing retention times to standards. The document outlines accepted chromatographic conditions and procedures A, B, and C described in pharmacopeias.Manufacturing operations and controls.pptx



Manufacturing operations and controls.pptxsaurabh11102000
Ěý
Chapter 5
As Per PCI Syllabus of
Quality Control and Quality Assurance
M.Pharm (Pharmaceutical Analysis)QUALIFICATION OF GAS CHROMATOGRAPHY.pptx



QUALIFICATION OF GAS CHROMATOGRAPHY.pptxSurendra Chowdary
Ěý
This document discusses the qualification of gas chromatography. It begins with an introduction to gas chromatography and its use in analytical chemistry. It then discusses the different types of qualifications including design qualification, installation qualification, operational qualification, and performance qualification. Design qualification ensures the instrument is designed properly. Installation qualification confirms proper installation. Operational qualification verifies the instrument operates as expected through tests of precision, accuracy, and other parameters. Performance qualification examines the instrument's ability to provide expected results. The document provides details on tests and acceptance criteria for each qualification type to ensure the gas chromatography instrument is functioning as intended.Validation qualification



Validation qualificationDevipriya Viswambharan
Ěý
This document discusses validation and qualification processes for pharmaceutical equipment and systems. It defines validation as providing objective evidence that a process meets its intended use consistently, while qualification ensures equipment is ready for its intended use. The document outlines the key steps in validation including developing a validation master plan, user requirement specification, design qualification, installation qualification, operational qualification and performance qualification. It provides details on each stage and emphasizes that validation and qualification are critical to ensuring product quality and compliance with cGMP regulations.Calibration vs Qualification



Calibration vs QualificationRaghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains Calibration vs Qualification and phases of qualification.
Presented by: A.Siddartha Tharun Teja. (Department of industrial pharmacy).
RIPER, anantapur.Concept & evolution of qa & qc



Concept & evolution of qa & qcChowdaryPavani
Ěý
QA and QC are related but distinct concepts in quality management. QA refers to the overall system that aims to prevent defects through processes, while QC tests products to identify defects. QA is a preventative system involving all employees to ensure quality standards are met throughout development. In contrast, QC is reactive and conducted by a specialized team to detect defects in finished products before release. Both work together to continually meet customer requirements, with QA focusing on building quality in from the start and QC checking for quality along the way.Qualification of high performance liquid chromatography



Qualification of high performance liquid chromatographysrikrupa institute of pharmaceutical analysis
Ěý
The document provides an overview of the qualification process for high performance liquid chromatography (HPLC) equipment, including design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). It describes the objectives and procedures for each qualification step. Key aspects covered include verifying design specifications, proper installation, operational requirements such as precision, accuracy and noise levels, and ongoing performance monitoring. The goal of qualification is to ensure analytical systems are suitable for their intended use and generate reliable results.Qualification of analytical instruments 



Qualification of analytical instruments PUNEET NIRMAL
Ěý
Brief Introduction of Analytical Instrument which are useful for Analysis in Pharma Field as well as Biotech Field.More Related Content
What's hot (20)
Calibration



CalibrationPradeep Kumar
Ěý
Calibration establishes the relationship between instrument measurements and known standard values through a series of steps. Key aspects of calibration include identifying instruments and sources, following calibration procedures, documenting results, accounting for sources of error, and ensuring traceability to national standards. Calibration procedures vary based on instrument type, but generally involve evaluating instrument performance, establishing calibration curves using certified reference materials at multiple concentration levels, and quantifying samples based on the calibration curves.PPT ON GOOD LABORATORY PRACTICES (GLP)



PPT ON GOOD LABORATORY PRACTICES (GLP)GOVIND YADAV
Ěý
The document discusses Good Laboratory Practice (GLP) guidelines. It notes that in the 1970s, the FDA discovered many cases of poor laboratory practices and fraudulent activities in the US. This led to the establishment of GLP principles to ensure reliable and high-quality non-clinical safety studies. GLP guidelines cover facilities, equipment, standard operating procedures, personnel training, record-keeping, and quality assurance programs. The goal of GLP is to ensure that experimental data accurately reflect the results and that studies are conducted in accordance with the principles of good laboratory practice.Analytical method validation as per ich and usp 



Analytical method validation as per ich and usp shreyas B R
Ěý
Analytical method validation is a process of documenting/ proving that an analytical method provides analytical data acceptable for the intended use.After the development of an analytical procedure, it is must important to assure that the procedure will consistently produce the intended a precise result with high degree of accuracy. The method should give a specific result that may not be affected by external matters. This creates a requirement to validate the analytical procedures. The validation procedures consists of some characteristics parameters that makes the method acceptable with addition of statistical tools.Qualification of analytical instruments



Qualification of analytical instrumentsFaris ameen
Ěý
This document provides guidelines for qualifying analytical instruments including electronic balances, pH meters, and UV-Visible spectrophotometers. It discusses the various levels of qualification including: Level I which involves selecting instruments and suppliers; Level II which involves installation and releasing instruments for use; Level III which involves periodic checks; and Level IV which involves in-use checks. Specific guidelines are provided for qualifying balances, pH meters, and UV-Visible spectrophotometers, including recommended tolerance limits for various parameters, calibration procedures, and qualification frequencies.Qualification of UV VISIBLE SPECTROPHOTOMETER



Qualification of UV VISIBLE SPECTROPHOTOMETERDr.K.Venkateswara raju
Ěý
This document discusses the qualification of UV-visible spectrophotometry. It begins by defining qualification as an act or process to ensure something complies with conditions, standards, or requirements. There are four types of qualification: design, installation, operational, and performance. UV-visible spectroscopy is concerned with the ultraviolet and visible regions ranging from 200-780 nm. The document outlines parameters for acceptance procedures and performance qualification of a UV-visible spectrophotometer, including wavelength accuracy, stray light, resolution power, noise, baseline flatness, stability, photometric accuracy, and linearity.Quality audit



Quality auditutkarsha shivsharan
Ěý
Quality audits involve the systematic and independent examination of quality activities and processes to determine compliance with established standards. They can be internal or external. The presented document discusses the definition, purpose, types, procedures, and important aspects of quality audits for pharmaceutical companies. It provides an overview of audit planning, conducting audits, common difficulties found, and essential features of audit reports. The goal is to evaluate procedures, records, and operations to ensure they meet quality goals and identify any non-compliances or deficiencies.calibration of analytical instruments 



calibration of analytical instruments Lavanya Pinneboina
Ěý
Calibration of analytical instruments is important to ensure they are accurate and precise. It involves comparing an instrument's measurements to a reference standard to determine any adjustments needed. Regular calibration helps verify that instruments are suitable for their intended purposes in pharmaceutical analysis. It is necessary to comply with quality standards and regulations. The calibration process involves using traceable standards and documented procedures to evaluate instruments and certify their performance is within specified tolerance limits. Maintaining calibration records provides a history of each instrument's accuracy over time.Validation master plan



Validation master planDr. Amsavel A
Ěý
The Validation Master Plan (VMP) outlines the company's approach to validation. It defines responsibilities, schedules, and documentation requirements for qualification of facilities, equipment, and processes. The VMP ensures management understands validation needs and the validation team understands their tasks. Key elements include qualification of equipment and facilities, process validation, cleaning validation, change control procedures, and periodic revalidation. Qualification includes design, installation, operational, and performance qualification to confirm equipment and facilities operate as intended. Process validation demonstrates manufacturing processes consistently produce products meeting specifications. The VMP helps regulatory inspectors evaluate the company's validation program.Qualification of GC & FTIR



Qualification of GC & FTIRMehulJain143
Ěý
The document provides details on qualification and validation procedures for gas chromatography (GC) and Fourier transform infrared spectroscopy (FTIR) systems used in pharmaceutical analysis. It describes calibration tests for both instruments, including tests to check wave number precision and reproducibility, linearity, and temperature accuracy and stability. Validation tests are also outlined, such as resolution checks and tests to evaluate performance against acceptance criteria over time as specified in guidance documents. The document provides a thorough overview of qualification processes for critical analytical instruments.Analytical method validation



Analytical method validationDevipriya Viswambharan
Ěý
This document discusses analytical method validation. It defines analytical method validation as providing assurance that an analytical method can consistently and accurately determine the presence or quantity of attributes. The objectives of validation are to obtain consistent, reliable and accurate data. Key parameters that are assessed in validation include specificity, accuracy, precision, linearity, range, limits of detection and quantification, ruggedness and robustness. The validation process involves planning, testing method performance characteristics, selecting validation acceptance criteria, and documenting results in a validation report. Validation is important for analytical methods used in pharmaceutical analysis.Basics of Impurity Profiling



Basics of Impurity ProfilingPankaj Soni
Ěý
describes what are impurities, sources of impurity, applications of impurity profiling and how to do impurity profiling.SITE ACCEPTANCE TEST



SITE ACCEPTANCE TESTRaghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains SITE ACCEPTANCE TEST IN QUALIFICATION.
Presented by: K. SANDHYA RANI (Department of pharmaceutical analysis ).RIPER, anantapurAnalytical method validation



Analytical method validationGaurav Kr
Ěý
This document discusses guidelines for analytical method validation. It outlines types of analytical methods that require validation including chromatographic, spectroscopic, and dissolution methods. Key analytical performance characteristics used in validation are described such as specificity, linearity, range, accuracy, precision, detection/quantitation limits, robustness, and system suitability testing. The document provides details on determining these characteristics and validating methods. It also addresses revalidation and references for further information.Validation Protocol



Validation ProtocolSagar K Savale
Ěý
ValidationĚýis a documented program that provides high degree of assurance that a specific process, method or system consistently produces a result meeting pre-determined acceptance criteria.Residual solvent 



Residual solvent Saptarshi Das
Ěý
This document summarizes the determination of residual solvents in pharmaceuticals by gas chromatography according to ICH guidelines. Residual solvents are organic chemicals used in manufacturing that are not fully removed and may pose health risks. They are classified into Class 1, 2, or 3 based on toxicity, with Class 1 solvents prohibited. Gas chromatography with headspace injection and flame ionization detection is the primary analytical method. It involves using capillary columns, temperature programs, and identifies solvents by comparing retention times to standards. The document outlines accepted chromatographic conditions and procedures A, B, and C described in pharmacopeias.Manufacturing operations and controls.pptx



Manufacturing operations and controls.pptxsaurabh11102000
Ěý
Chapter 5
As Per PCI Syllabus of
Quality Control and Quality Assurance
M.Pharm (Pharmaceutical Analysis)QUALIFICATION OF GAS CHROMATOGRAPHY.pptx



QUALIFICATION OF GAS CHROMATOGRAPHY.pptxSurendra Chowdary
Ěý
This document discusses the qualification of gas chromatography. It begins with an introduction to gas chromatography and its use in analytical chemistry. It then discusses the different types of qualifications including design qualification, installation qualification, operational qualification, and performance qualification. Design qualification ensures the instrument is designed properly. Installation qualification confirms proper installation. Operational qualification verifies the instrument operates as expected through tests of precision, accuracy, and other parameters. Performance qualification examines the instrument's ability to provide expected results. The document provides details on tests and acceptance criteria for each qualification type to ensure the gas chromatography instrument is functioning as intended.Validation qualification



Validation qualificationDevipriya Viswambharan
Ěý
This document discusses validation and qualification processes for pharmaceutical equipment and systems. It defines validation as providing objective evidence that a process meets its intended use consistently, while qualification ensures equipment is ready for its intended use. The document outlines the key steps in validation including developing a validation master plan, user requirement specification, design qualification, installation qualification, operational qualification and performance qualification. It provides details on each stage and emphasizes that validation and qualification are critical to ensuring product quality and compliance with cGMP regulations.Calibration vs Qualification



Calibration vs QualificationRaghavendra institute of pharmaceutical education and research .
Ěý
In this slide contains Calibration vs Qualification and phases of qualification.
Presented by: A.Siddartha Tharun Teja. (Department of industrial pharmacy).
RIPER, anantapur.Concept & evolution of qa & qc



Concept & evolution of qa & qcChowdaryPavani
Ěý
QA and QC are related but distinct concepts in quality management. QA refers to the overall system that aims to prevent defects through processes, while QC tests products to identify defects. QA is a preventative system involving all employees to ensure quality standards are met throughout development. In contrast, QC is reactive and conducted by a specialized team to detect defects in finished products before release. Both work together to continually meet customer requirements, with QA focusing on building quality in from the start and QC checking for quality along the way.Similar to Calibration (20)
Qualification of high performance liquid chromatography



Qualification of high performance liquid chromatographysrikrupa institute of pharmaceutical analysis
Ěý
The document provides an overview of the qualification process for high performance liquid chromatography (HPLC) equipment, including design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). It describes the objectives and procedures for each qualification step. Key aspects covered include verifying design specifications, proper installation, operational requirements such as precision, accuracy and noise levels, and ongoing performance monitoring. The goal of qualification is to ensure analytical systems are suitable for their intended use and generate reliable results.Qualification of analytical instruments 



Qualification of analytical instruments PUNEET NIRMAL
Ěý
Brief Introduction of Analytical Instrument which are useful for Analysis in Pharma Field as well as Biotech Field.Clinical lab qc sethu



Clinical lab qc sethusubramaniam sethupathy
Ěý
This document discusses quality control in clinical laboratory testing. It emphasizes that quality control is essential to provide reliable diagnostic reports and cost-effective patient care. Quality control involves monitoring precision, accuracy, and sources of variation through internal quality control, external quality assessment, and statistical analysis of control values using tools like control charts and Westgard rules. The goal is to minimize laboratory errors and reliably distinguish pathological variations in patient samples.Assuring of Test Results based on Statistical tools.pptx



Assuring of Test Results based on Statistical tools.pptxasresmekonen
Ěý
for Physico chemical laboratoryPHARMACEUTICAL CALIBRATION, QUALIFICATION AND VALIDATION: AN INTRODUCTION



PHARMACEUTICAL CALIBRATION, QUALIFICATION AND VALIDATION: AN INTRODUCTIONPrincy Agarwal
Ěý
A presentation on introduction of calibration, qualification and validation in pharmaceutical industries.Key steps in transforming a calibration program slideshare



Key steps in transforming a calibration program slideshareJohn Cummins, CPIP
Ěý
An overview of how to take a helicopter view of calibration programs and implement a step change in performance. Pharmaceutical Instrument and Analytical Validation and Qualification (SHOPNI...



Pharmaceutical Instrument and Analytical Validation and Qualification (SHOPNI...ShopnilAkash5
Ěý
ValidationĚýis the process of establishing documentary evidence demonstrating that a procedure, process, or activity carried out in testing and then production maintains the desired level of compliance at all stages
It is essential to ensure the quality Of a product and Good Manufacturing Practices including all other regulatory requirements
Qualification of instruments



Qualification of instrumentsNavdha Soni
Ěý
This document provides guidelines for qualification of analytical balances and pH meters used in official medicines control laboratories (OMCLs). It discusses the requirements for selection, installation, calibration and ongoing testing of balances and pH meters. Key points include checking that balances are installed in stable, vibration-free locations with controlled temperature and humidity; calibrating balances and pH meters upon installation and on a defined frequency; and conducting tests to verify accuracy, precision, linearity and other performance characteristics. Proper management and calibration of pH electrodes is also covered. The document aims to ensure analytical instruments are qualified and meet performance standards for their intended use in chemical and biological testing.calibrationintervalspresentation.pdf



calibrationintervalspresentation.pdfAbdulSalamSagir1
Ěý
The document discusses factors to consider when selecting appropriate calibration intervals for measuring instruments. It explains that calibration intervals should account for the instrument type, usage conditions, measurement uncertainty requirements, and risk of exceeding error limits. The document reviews various interval methods including fixed intervals, automatic adjustment based on past calibration results, in-use time tracking, and control charts which can help predict when recalibration is needed to maintain control. Selecting calibration intervals involves balancing calibration frequency against costs while ensuring measurement accuracy and quality.ICP QC protocol



ICP QC protocolGamal Abdel Hamid
Ěý
The analyst is required to analyze a number of QC samples throughout the run where there are decisions to be made based on a window of acceptance for each QC sample analyzed.
12. QA & QC Conventional and Digital Radiography by Ravindra Kumar .pptx



12. QA & QC Conventional and Digital Radiography by Ravindra Kumar .pptxRavindra Kumar
Ěý
Radiology's contribution to better diagnosis and treatments is evident. In parallel, efforts were oriented towards improving and controlling equipment.
The importance of quality assurance (QA) of diagnostic X-ray equipment is well recognized. Application of QA program is very important when optimization of image quality and reduction of patient exposure is desired.
Quality Assurance (QA) of medical diagnostic x-ray equipment means systematic actions necessary to provide adequate confidence to the end-user(s) that a medical diagnostic x-ray equipment will perform satisfactory in compliance with safety standards specified by the Competent Authority.
The goal of QA Program is to ensure the accuracy of the diagnosis. The purpose of Quality assurance and Quality control is to
1. Minimize the radiation dose to the patient
2. Increasing the life span of the equipment
3. Provide maximum good quality images.
The Quality Control (QC) is a central part of QA program, which deals with Equipment Maintenance and Monitoring.
1. Responsibility
2. Evaluations
3. Purchase specifications
4. Standards for Image Quality
5. Monitoring and Maintenance
6. Training
7. Committee
8. Records
9. Manual
10. Review.
QA in Digital Radiography: Digital radiography systems have replaced traditional analogue or screen-film systems.
CR System: CR technology uses an indirect conversion process using a two-stage technique.
DR System: Direct-conversion detectors have an X-ray photoconductor, such as amorphous selenium (a-Se), that converts directly at only one stage X-ray photons into electric charges.
Indirect-conversion systems use a two-stage technique for conversion. They have a scintillator, such as caesium iodide (CsI) that converts X-rays into visible light at the first stage.
Quality Assurance Test in CR System :
A. Daily Tests :
Image Artifact Test
Reader Reboot
Image Reader Visual Check
Inspection & Cleaning
B. Quarterly Tests :
Laser Jitter Test
Imaging Plate Cleaning
Retake Image analysis
C. Annual Test :
IP Dark Noise Test
Uniformity Test.
Regulatory Bodies for Equipment Standards :
*The Bureau of Indian Standards (BIS) is the national Standards Body of India working under the Ministry of Consumer Affairs, Food & Public Distribution, Government of India.
These include representatives of various interests such as consumers, regulatory and other Government bodies, industry, scientists, technologists, testing organizations and consultants.
The manufacturers of diagnostic x-ray machines in the country are directed that each make/model of x-ray unit manufactured by them shall bear certification mark of the BIS issued by BIS.
Digital radiography systems have been replacing the screen film system.
Digital radiology requires some specific training to benefit of this new technique.
Image quality and diagnostic information are closely related to patient dose.
Quality assurance programs are essential in digital radiology due to the risk of increase.Validation of lab instruments and quantitative test methods 



Validation of lab instruments and quantitative test methods Mostafa Mahmoud
Ěý
This lecture shows the procedures applied when going to validate your laboratory instruments and quantitative test methods also either FDA approved or laboratory developed tests.Acceptance sampling



Acceptance samplingHassan Habib
Ěý
The presentation depicted herein presents briefly an introduction of acceptance sampling along with some major differences amongst the widely used sampling standards.
Acceptance Sampling standards comparison. MIL-STD-105E, MIL-STD-1916, ISO 2859, ISO 3951. About AQLs and OC Curves.Quality assurance in a medical laboratory



Quality assurance in a medical laboratoryCHRISTIAN MEDICAL COLLEGE AND HOSPITAL
Ěý
A routine session on quality assurance practice in a medical laboratory to sensitize and provide basics to those interested in working in a medical testing laboratory. Presentation for APPON



Presentation for APPONSushan Shrestha
Ěý
Caltech Private Limited held a meeting to discuss calibration. They explained that calibration verifies the accuracy of instruments by comparing them to standard references. It is important to calibrate regularly to avoid false test results. The benefits of calibration include ensuring accuracy, minimizing breakdowns, and meeting certification requirements. Calibration is also important for quality improvement, safety, and interrelating quality and productivity. Caltech recommends on-site calibration for pharmaceuticals. They provide quality calibration services with accredited equipment and traceable standards.Calibration



CalibrationAsep Herman
Ěý
The document discusses calibration procedures and methodologies. It outlines the objectives of calibration to ensure accurate measurements over time despite wear and involves calibrating all measuring equipment. A calibration hierarchy is described from primary standards to reference to working standards. Factors that affect calibration such as environment, standard accuracy, out of tolerance equipment, traceability, and labeling are also covered.Quality Control in Laboratory



Quality Control in LaboratoryManal Elsayed CPPS, CPHQ, CLSSBB, FISQua, DTQM
Ěý
This document discusses quality control in laboratories. It defines key terms like quality assurance, quality assessment, total quality management, and continuous quality improvement. It describes factors that can affect quality like pre-analytical, analytical, and post-analytical variables. The importance of standard operating procedures, proficiency testing, and documenting quality control procedures is emphasized. Maintaining accurate and precise results through internal quality control using control charts and Westgard rules is also outlined.QUALIFICATION OF HPLC , HPLC



QUALIFICATION OF HPLC , HPLCsuriyapriya kamaraj
Ěý
This presentation explains about qualifications of HPTLC, types of qualifications, design qualification , installation qualification ,operational qualification, performance qualification ,documentation of qualification .QA QC Program for Waste Water Analysis ppt



QA QC Program for Waste Water Analysis pptKrisnaBagtasos1
Ěý
This document discusses quality assurance and quality control programs for waste water analysis laboratories. It outlines key differences between quality assurance and quality control such as their focus on process vs. product and being proactive vs. reactive. It also provides details on quality assurance documentation and measures including calibration, reference materials, and proficiency testing. For quality control, it describes validation and verification of analytical methods, determination of method detection limits, initial and ongoing demonstration of analyst capability, control charts, and corrective actions. Specific procedures are defined for determining method detection limits, accuracy, precision, linearity, and reporting verification results.Qualification of high performance liquid chromatography



Qualification of high performance liquid chromatographysrikrupa institute of pharmaceutical analysis
Ěý
Recently uploaded (20)
How to Manage Putaway Rule in Odoo 17 Inventory



How to Manage Putaway Rule in Odoo 17 InventoryCeline George
Ěý
Inventory management is a critical aspect of any business involved in manufacturing or selling products.
Odoo 17 offers a robust inventory management system that can handle complex operations and optimize warehouse efficiency. Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...



Blind Spots in AI and Formulation Science Knowledge Pyramid (Updated Perspect...Ajaz Hussain
Ěý
This presentation delves into the systemic blind spots within pharmaceutical science and regulatory systems, emphasizing the significance of "inactive ingredients" and their influence on therapeutic equivalence. These blind spots, indicative of normalized systemic failures, go beyond mere chance occurrences and are ingrained deeply enough to compromise decision-making processes and erode trust.
Historical instances like the 1938 FD&C Act and the Generic Drug Scandals underscore how crisis-triggered reforms often fail to address the fundamental issues, perpetuating inefficiencies and hazards.
The narrative advocates a shift from reactive crisis management to proactive, adaptable systems prioritizing continuous enhancement. Key hurdles involve challenging outdated assumptions regarding bioavailability, inadequately funded research ventures, and the impact of vague language in regulatory frameworks.
The rise of large language models (LLMs) presents promising solutions, albeit with accompanying risks necessitating thorough validation and seamless integration.
Tackling these blind spots demands a holistic approach, embracing adaptive learning and a steadfast commitment to self-improvement. By nurturing curiosity, refining regulatory terminology, and judiciously harnessing new technologies, the pharmaceutical sector can progress towards better public health service delivery and ensure the safety, efficacy, and real-world impact of drug products.EDL 290F Week 3 - Mountaintop Views (2025).pdf



EDL 290F Week 3 - Mountaintop Views (2025).pdfLiz Walsh-Trevino
Ěý
EDL 290F Week 3 - Mountaintop Views (2025).pdfResearch & Research Methods: Basic Concepts and Types.pptx



Research & Research Methods: Basic Concepts and Types.pptxDr. Sarita Anand
Ěý
This ppt has been made for the students pursuing PG in social science and humanities like M.Ed., M.A. (Education), Ph.D. Scholars. It will be also beneficial for the teachers and other faculty members interested in research and teaching research concepts.Digital Tools with AI for e-Content Development.pptx



Digital Tools with AI for e-Content Development.pptxDr. Sarita Anand
Ěý
This ppt is useful for not only for B.Ed., M.Ed., M.A. (Education) or any other PG level students or Ph.D. scholars but also for the school, college and university teachers who are interested to prepare an e-content with AI for their students and others.CBSE Arabic Grammar - Class 10 ppt.pptx



CBSE Arabic Grammar - Class 10 ppt.pptxsuhail849886
Ěý
cbse arabic grammar
grade 10 cbse arabic grammar
cbse class 10 arabic grammar
arabic marathon cbse arabic 10
nominal sentences
FESTIVAL: SINULOG & THINGYAN-LESSON 4.pptx



FESTIVAL: SINULOG & THINGYAN-LESSON 4.pptxDanmarieMuli1
Ěý
Sinulog Festival of Cebu City, and Thingyan Festival of Myanmar.How to use Init Hooks in Odoo 18 - Odoo şÝşÝߣs



How to use Init Hooks in Odoo 18 - Odoo şÝşÝߣsCeline George
Ěý
In this slide, we’ll discuss on how to use Init Hooks in Odoo 18. In Odoo, Init Hooks are essential functions specified as strings in the __init__ file of a module.Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Prelims of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Kaun TALHA quiz Finals -- El Dorado 2025



Kaun TALHA quiz Finals -- El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Finals of Kaun TALHA : a Travel, Architecture, Lifestyle, Heritage and Activism quiz, organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. Mate, a short story by Kate Grenville.pptx



Mate, a short story by Kate Grenville.pptxLiny Jenifer
Ěý
A powerpoint presentation on the short story Mate by Kate Greenville. This presentation provides information on Kate Greenville, a character list, plot summary and critical analysis of the short story.Database population in Odoo 18 - Odoo slides



Database population in Odoo 18 - Odoo slidesCeline George
Ěý
In this slide, we’ll discuss the database population in Odoo 18. In Odoo, performance analysis of the source code is more important. Database population is one of the methods used to analyze the performance of our code. Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Finals of Rass MELAI : a Music, Entertainment, Literature, Arts and Internet Culture Quiz organized by Conquiztadors, the Quiz society of Sri Venkateswara College under their annual quizzing fest El Dorado 2025. How to attach file using upload button Odoo 18



How to attach file using upload button Odoo 18Celine George
Ěý
In this slide, we’ll discuss on how to attach file using upload button Odoo 18. Odoo features a dedicated model, 'ir.attachments,' designed for storing attachments submitted by end users. We can see the process of utilizing the 'ir.attachments' model to enable file uploads through web forms in this slide.Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Prelims - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Kaun TALHA quiz Finals -- El Dorado 2025



Kaun TALHA quiz Finals -- El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025



Rass MELAI : an Internet MELA Quiz Finals - El Dorado 2025Conquiztadors- the Quiz Society of Sri Venkateswara College
Ěý
Calibration
- 1. CALIBRATION Ms.R.L. Mhetre Asst. Professor Modern College of Pharmacy, Moshi, Pune
- 2. CALIBRATION: It is comparison between the reference standard and instrument to be calibrated. Reference standard material used for calibration should be calibrated according to ISO/IEC 17025. GOAL: • TO MINIMIZE ANY MEASUREMENT UNCERTAINITY BY ENSURING THE ACCURACY OF THE TEST EQUIPMENT. • CALIBRATION QUALIFIES AND CONTROLS ERRORS OR UNCERTAINITIES WITHIN MEASUREMENT PROCESS TO AN ACCEPTABLE LEVEL. • TO DETERMINE WHETHER EQUIPMENT IS STILL FIT FOR ITS INTENDED USE. Where to be calibrate • I/II/III PARTY LABORATORY • ACCREDITED CALIBRATION LABPRATORY- MANUFACTURER/SUPPLIER LABORATORY.
- 3. BASIC REQUIRMENT OF CALIBRATION • Location: Permanent • Environment: temp, humidity, airflow, filtration, noise level, vibrations, cleanliness, power supply, calibartion areas • Equipment: Reference standard, working standard, accessories, computer system, software • Staff: training, responsibility, technical competence, authority • Management system: document, calibration record.
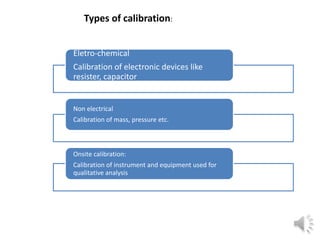
- 4. Types of calibration: Eletro-chemical Calibration of electronic devices like resister, capacitor Non electrical Calibration of mass, pressure etc. Onsite calibration: Calibration of instrument and equipment used for qualitative analysis
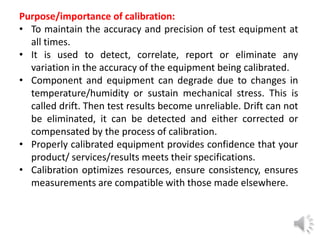
- 5. Purpose/importance of calibration: • To maintain the accuracy and precision of test equipment at all times. • It is used to detect, correlate, report or eliminate any variation in the accuracy of the equipment being calibrated. • Component and equipment can degrade due to changes in temperature/humidity or sustain mechanical stress. This is called drift. Then test results become unreliable. Drift can not be eliminated, it can be detected and either corrected or compensated by the process of calibration. • Properly calibrated equipment provides confidence that your product/ services/results meets their specifications. • Calibration optimizes resources, ensure consistency, ensures measurements are compatible with those made elsewhere.
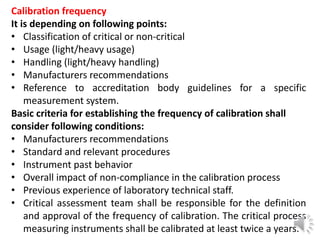
- 6. Calibration frequency It is depending on following points: • Classification of critical or non-critical • Usage (light/heavy usage) • Handling (light/heavy handling) • Manufacturers recommendations • Reference to accreditation body guidelines for a specific measurement system. Basic criteria for establishing the frequency of calibration shall consider following conditions: • Manufacturers recommendations • Standard and relevant procedures • Instrument past behavior • Overall impact of non-compliance in the calibration process • Previous experience of laboratory technical staff. • Critical assessment team shall be responsible for the definition and approval of the frequency of calibration. The critical process measuring instruments shall be calibrated at least twice a years.
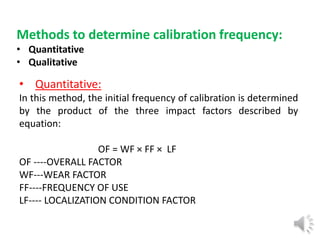
- 7. Methods to determine calibration frequency: • Quantitative • Qualitative • Quantitative: In this method, the initial frequency of calibration is determined by the product of the three impact factors described by equation: OF = WF × FF × LF OF ----OVERALL FACTOR WF---WEAR FACTOR FF----FREQUENCY OF USE LF---- LOCALIZATION CONDITION FACTOR
- 8. WF FF LF High= 9 or 10 High= 9 or 10 Every day= 9 or 10 Moderate = 6, 7 or 8 Moderate = 6, 7 or 8 Every week = 6, 7 or 8 Low = 3,4 or 5 Low = 3,4 or 5 Every month= 3, 4 or 5 Very low= 1 or 2 Very low= 1 or 2 Every year or twice a year= 1 or 2
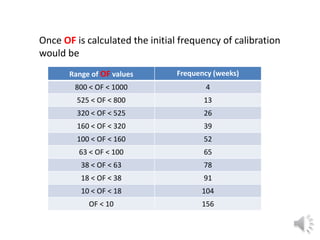
- 9. Once OF is calculated the initial frequency of calibration would be Range of OF values Frequency (weeks) 800 < OF < 1000 4 525 < OF < 800 13 320 < OF < 525 26 160 < OF < 320 39 100 < OF < 160 52 63 < OF < 100 65 38 < OF < 63 78 18 < OF < 38 91 10 < OF < 18 104 OF < 10 156
- 10. QUALITATIVE: Here, the initial frequency of calibration is selected according to the following points: 1. Characteristics: Degree of importance of the variable to be controlled to ensure the quality of product to be manufactured and it can be designed as critical, significant, important or not special. 2. Intensity of use: Rare, often and quiet often. 3. Environment: Loss of calibration due to different operating conditions is a critical factor to be considered in the definition of the frequency of calibration. These factors can be classifies as under control, moderate and aggressive.
- 11. Thank you






