Carbon and its importance by jeswant gembali
- 1. By- Jeswant GembaliBy- Jeswant Gembali
- 2. Position of Carbon in Periodic TableŌĆ”ŌĆ”ŌĆ”ŌĆ”.
- 3. Carbon AtomŌĆÖs Electronic structure L-shell (4 e- ) K-shell (2 e- ) Nucleus 6 Neutrons & 6 Protons Atomic No. 6
- 4. Occurrence of Carbon in Nature :- ’é× In free state:- ’é× In Combined state:- CO2 CO3
- 5. Carbon in daily life:-
- 6. Importance of Carbon:- ’é× TetravalencyŌĆ”.. Atomic No. :- 6 Electronic Configuration :- 2,4 Thus, Valency :- 4 c . .. .
- 7. Importance of Carbon:- ’é× Multiple Bonds c
- 8. Importance of Carbon:- ’é× Catenation (Self- linkage)
- 9. Importance of Carbon:- ’é× Isomerism (Property of making Isomers):- Isomers; Same Molecular Formula but different Structure. For eg:- C6H12O6 (Glucose) C6H12O6 (Fructose) Glucose Fructose
- 10. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Diamond Properties:- ’üČ Excellent Brilliance. ’üČ A Transparent Object. ’üČ Bad Conductor of Electricity. ’üČ High Melting Point . ’üČ Made up of Carbon atoms only. ’üČ Hardest substance in the World. ’üČ Can be cut with the help of Another Diamond only.
- 11. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Diamond Structure:- ’üČ Tetrahedral structure. ’üČ 1C linked with 4C ’üČ Linked with strong Covalent bonds. ’üČ A Continuous chain of Carbon atoms.
- 12. Uses of Diamond
- 13. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Graphite Properties:- ’üČ An Opaque Object. ’üČ Good Conductor of Electricity. ’üČ High Melting Point . ’üČ Made up of Carbon atoms only. ’üČ Liberates Carbon dioxide when heated.
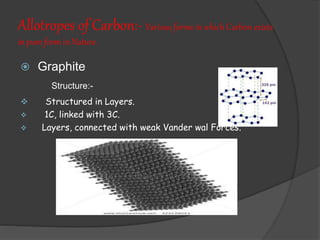
- 14. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Graphite Structure:- ’üČ Structured in Layers. ’üČ 1C, linked with 3C. ’üČ Layers, connected with weak Vander wal Forces.
- 15. Graphite in Daily │óŠ▒┤┌▒ŌĆ”.
- 16. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Buckminster fullerene Properties:- ’üČ Black solid at room temperature. ’üČ DoesnŌĆÖt conducts electricity. ’üČ Insoluble in Water. ’üČ Soluble in Petrol. * Has been named after a great American Architect ŌĆ£BuckminsterŌĆØ
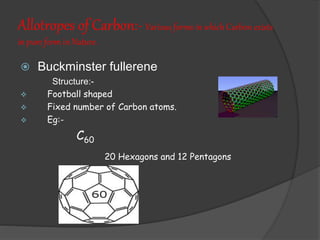
- 17. Allotropes of Carbon:- Various forms in which Carbon exists in pure form in Nature. ’é× Buckminster fullerene Structure:- ’üČ Football shaped ’üČ Fixed number of Carbon atoms. ’üČ Eg:- C60 20 Hexagons and 12 Pentagons