Catalysis
- 1. 1
- 2. ÔÇß Transition metal catalysed reactions ÔÇß Organo-catalysis in organic synthesis ÔÇß Bio catalysis ÔÇß Phase transfer catalysis 2
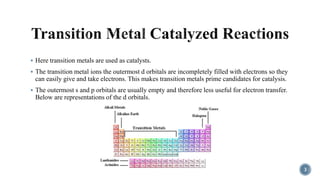
- 3. ÔÇß Here transition metals are used as catalysts. ÔÇß The transition metal ions the outermost d orbitals are incompletely filled with electrons so they can easily give and take electrons. This makes transition metals prime candidates for catalysis. ÔÇß The outermost s and p orbitals are usually empty and therefore less useful for electron transfer. Below are representations of the d orbitals. 3
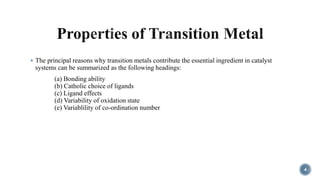
- 4. ÔÇß The principal reasons why transition metals contribute the essential ingredient in catalyst systems can be summarized as the following headings: (a) Bonding ability (b) Catholic choice of ligands (c) Ligand effects (d) Variability of oxidation state (e) Variablility of co-ordination number 4
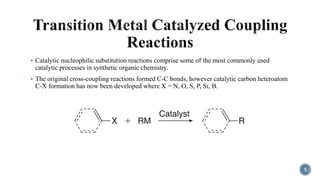
- 5. ÔÇß Catalytic nucleophilic substitution reactions comprise some of the most commonly used catalytic processes in synthetic organic chemistry. ÔÇß The original cross-coupling reactions formed C-C bonds, however catalytic carbon heteroatom C-X formation has now been developed where X = N, O, S, P, Si, B. 5
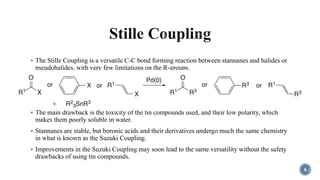
- 6. 6 ÔÇß The Stille Coupling is a versatile C-C bond forming reaction between stannanes and halides or pseudohalides, with very few limitations on the R-groups. ÔÇß The main drawback is the toxicity of the tin compounds used, and their low polarity, which makes them poorly soluble in water. ÔÇß Stannanes are stable, but boronic acids and their derivatives undergo much the same chemistry in what is known as the Suzuki Coupling. ÔÇß Improvements in the Suzuki Coupling may soon lead to the same versatility without the safety drawbacks of using tin compounds.
- 7. 7
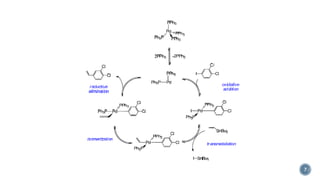
- 8. ÔÇß The coupling of organoboron reagents has become the most commonly used cross-coupling process. Organoboron reagents are less toxic than organotin reagents and tend to undergo coupling reactions in the presence of a variety of functional groups. ÔÇß Like neutral organosilicon groups (Denmark rxn), however, neutral organoboron reagents do not undergo metal-catalyzed cross-coupling without an additive. ÔÇß Suzuki showed that addition of a hard base, e.g. OH‚àí or F‚àí , causes the organoboron reagent to undergo cross-coupling by generating a four-coordinate anionic organoboron reagent that transfers the organic group from boron to the metal catalyst. ÔÇß The scheme below shows the first published Suzuki Coupling, which is the palladium catalysed cross coupling between organoboronic acid and halides. 8
- 9.  The term Organo catalysis refers to a form of catalysis, whereby the rate of a chemical reaction is increased by an organic catalyst referred to as an “Organo catalyst" consisting of carbon, hydrogen, sulfur and other nonmetal elements found in organic compounds.  Regular achiral organo catalysts are based on nitrogen such as piperidine used in the Knoevenagel condensation. DMAP used in esterification and DABCO used in the Baylis- Hillman reaction. Thiazolium salts are employed in the Stetter reaction. These catalysts and reactions have a long history but current interest in organo catalysis is focused on asymmetric catalysis with chiral catalysts, called asymmetric organo catalysis or enantioselective organo catalysis. 9
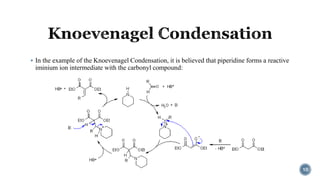
- 10. ÔÇß In the example of the Knoevenagel Condensation, it is believed that piperidine forms a reactive iminium ion intermediate with the carbonyl compound: 10
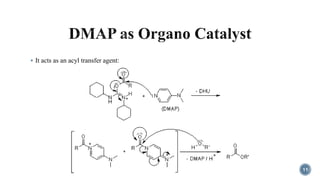
- 11. ÔÇß It acts as an acyl transfer agent: 11
- 12. ÔÇß A catalyst is a substance which alters to promote the reaction and a substance especially an enzyme, that initiates or modifies the rate of a chemical reaction in a living body is termed as biocatalyst. ÔÇß They are enzymes or microbes that initiate or accelerate chemical reactions. 12
- 13.  Dehydrogenases  NAD(P)H-dependent dehydrogenases for the asymmetric reduction of ketones, ketoacids, olefins etc.…  Oxidation of alcohols with dehydrogenases  Oxygenases  Monohydroxylations, especially for the hydroxylation of non-activated centers and of non-natural substrates. Improve practicability, robustness and substrate scope of the in vitro CYP450s systems, develop an FMO- based alternative system  Transformation of ribonucleotides, stereospecific epoxidations, oxidation of ketones to esters and lactones.  Lyases  Synthetically useful enzymes for C–C bond formation (preferably asymmetric) using aldolases, hydroxynitrile lyases and ThDP-dependent lyases  C–N (aminolyases) and C–O (hydratases) bond formations. Identification of lyases with a broad substrate acceptance 13
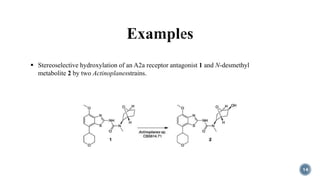
- 14. 14 ÔÇß Stereoselective hydroxylation of an A2a receptor antagonist 1 and N-desmethyl metabolite 2 by two Actinoplanesstrains.
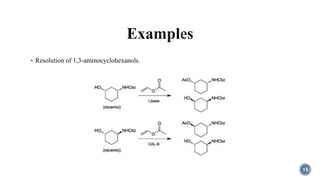
- 15. ÔÇß Resolution of 1,3-aminocyclohexanols. 15
- 16. ÔÇß Synthesis of (1S,4R)-cis-4-acetoxy-2-cyclopentene-1-ol by lipase-catalyzed hydrolytic desymmetrization. 16
- 17. ÔÇß In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. ÔÇß Phase-transfer catalysis is a special form of heterogeneous catalysis. ÔÇß Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst ÔÇß The catalyst functions like a detergent for solubilizing the salts into the organic phase. 17
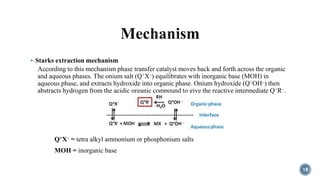
- 18.  Starks extraction mechanism According to this mechanism phase transfer catalyst moves back and forth across the organic and aqueous phases. The onium salt (Q+X–) equilibrates with inorganic base (MOH) in aqueous phase, and extracts hydroxide into organic phase. Onium hydroxide (Q+OH–) then abstracts hydrogen from the acidic organic compound to give the reactive intermediate Q+R–. Q+X– = tetra alkyl ammonium or phosphonium salts MOH = inorganic base 18
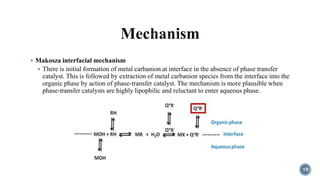
- 19. ÔÇß Makosza interfacial mechanism ÔÇß There is initial formation of metal carbanion at interface in the absence of phase transfer catalyst. This is followed by extraction of metal carbanion species from the interface into the organic phase by action of phase-transfer catalyst. The mechanism is more plausible when phase-transfer catalysts are highly lipophilic and reluctant to enter aqueous phase. 19
- 20.  The phase transfer catalytic processes can be categorized as follows depending on the number of phases involved. i. liquid–liquid phase transfer catalysis ii. solid–liquid phase transfer catalysis iii. third-liquid phase-transfer catalysis 20
- 21.  The solid-liquid PTC usually involves reaction of an anionic reagent in a solid phase, with a reactant located in contiguous liquid organic phase.  In solid-liquid PTC, the first step involves the transport of a reactant anion from the solid phase to the organic phase by a phase-transfer cation.  The second step involves the reaction of the transferred anion with the reactant located in the organic phase.  Solid – liquid PTC are used for alkylation of highly acidic compound, preparation of amino acids or aldol-type condensation.  The process of hydroperoxide acylation in presence of anhydrous Na2CO3using solid – liquid PTC system can be demonstrated by a sequence of the following reactions - 21
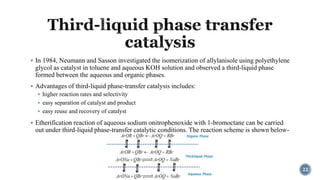
- 22. ÔÇß In 1984, Neumann and Sasson investigated the isomerization of allylanisole using polyethylene glycol as catalyst in toluene and aqueous KOH solution and observed a third-liquid phase formed between the aqueous and organic phases. ÔÇß Advantages of third-liquid phase-transfer catalysis includes: ÔÇß higher reaction rates and selectivity ÔÇß easy separation of catalyst and product ÔÇß easy reuse and recovery of catalyst ÔÇß Etherification reaction of aqueous sodium onitrophenoxide with 1-bromoctane can be carried out under third-liquid phase-transfer catalytic conditions. The reaction scheme is shown below- 22
- 23. 23
- 24.  Meyer H, Eichhorn E, Hanlon S, Lütz S, Schürmann M, Wohlgemuth R et al. The use of enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Catal Sci Technol. 2013;3(1):29-40.  Clayden J, Greeves N, Warren S. Organic Chemistry. 2nd ed. New Delhi: Oxford University Press; 2001.  Phase Transfer Catalysis [Internet]. NPTEL. 2014 [cited 30 July 2014]. Available from: http://nptel.ac.in/courses/103103026/44 24
- 25. 25









![ Meyer H, Eichhorn E, Hanlon S, Lütz S, Schürmann M, Wohlgemuth R et al. The use of
enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial
Biocatalysis Consortium (SIBC). Catal Sci Technol. 2013;3(1):29-40.
ÔÇß Clayden J, Greeves N, Warren S. Organic Chemistry. 2nd ed. New Delhi: Oxford University
Press; 2001.
ÔÇß Phase Transfer Catalysis [Internet]. NPTEL. 2014 [cited 30 July 2014]. Available from:
http://nptel.ac.in/courses/103103026/44
24](https://image.slidesharecdn.com/catalysis-180419055602/85/Catalysis-24-320.jpg)







![Homogeneous catalysis [ MPHARM, MSC, BPHARM, BSC]](https://cdn.slidesharecdn.com/ss_thumbnails/62-191219155346-thumbnail.jpg?width=560&fit=bounds)



















![HETEROGENOUS CATALYSIS [CHEMISTRY IS LOVE]](https://cdn.slidesharecdn.com/ss_thumbnails/50-191219085938-thumbnail.jpg?width=560&fit=bounds)





![Catalysis by solid bases [recovered] [autosaved]](https://cdn.slidesharecdn.com/ss_thumbnails/catalysisbysolidbasesrecoveredautosaved-210612140910-thumbnail.jpg?width=560&fit=bounds)


































