Ch 1.pdf
- 1. PHILADELPHIA UNIVERSITY Department of Civil Engineering Hydraulics (670441) CHAPTER 1 Fundamental Properties of Water Instructor: Eng. Abdallah Odeibat Civil Engineer, Structures , M.Sc. 1
- 2. ’éó The word hydraulic comes from two Greek words: "hydor" (meaning water) and "aulos" (meaning pipe). Through the years, the definition of hydraulics has broadened beyond merely pipe flow. Hydraulic systems are designed to accommodate water at rest and in motion. ’éó The fundamentals of hydraulic engineering systems, therefore, involve the application of engineering principles and methods to the planning, control, transportation, conservation, and utilization of water. 2
- 3. 1.1 THE EARTH'S ATMOSPHERE AND ATMOSPHERIC PRESSURE ’éó The earthŌĆÖs atmosphere is a thick layer (approximately 1,500 km) of mixed gases. Nitrogen makes up approximately 78 percent of the atmosphere, oxygen makes up approximately 21 percent, and the remaining 1 percent consists mainly of water vapor, argon, and trace amounts of other gases. ’éó Each gas possesses a certain mass and consequently has a weight. The total weight of the atmospheric column exerts a pressure on every surface it contacts. At sea level and under normal conditions, the atmospheric pressure is approximately equal to 1.014 *105 N/m2, or approximately 1 bar. 3
- 4. ’éó The pressure unit 1 N/m2 is also known as 1 pascal, named after French mathematician Blaise Pascal (1623 to 1662). ’éó Water surfaces that come in contact with the atmosphere are subjected to atmospheric pressure. In the atmosphere, each gas exerts a partial pressure independently of the other gases. The partial pressure exerted by the water vapor in the atmosphere is called the vapor pressure. 4
- 5. 1.2 THE THREE PHASES OF WATER ’éó The water molecule is a stable chemical bond of oxygen and hydrogen atoms. The amount of energy holding the molecules together depends on the temperature and pressure present. Depending on its energy content, water may appear in solid, liquid, or gaseous form. ’éó Snow and ice are the solid forms of water; liquid is its most commonly recognized form; and moisture, water vapor in air, is water in its gaseous form. The three different forms of water are called its three phases. ’éó To change water from one phase to another phase, energy must either be added or taken away from the water. The amount of energy required to change water from one phase to another is known as a latent energy. This amount of energy may be in the form of heat or pressure. One of the most common units of heat energy is the calorie (cal). 5
- 6. ’éó One calorie is the energy required to increase the temperature of 1 gram (g) of water, in liquid phase, by 1┬░C. The amount of energy required to raise the temperature of a substance by 1┬░C is known as the specific heat of that substance. ’éó Under standard atmospheric pressure, the specific heat of water and ice are, respectively, 1.0 and 0.465 cal/g.┬░C. For water vapor, the specific heat under constant pressure is 0.432 cal/g.┬░C, and at constant volume it is 0.322 cal/g.┬░C. ’éó These values may vary slightly depending on the purity of the water. To melt 1 g of ice, changing water from its solid to liquid phase, requires a latent heat (heat of fusion) of 79.7 cal. To freeze water, the same amount of heat energy must be taken out of each of water, thus the process is reversed. 6
- 7. ’éó Evaporation, the changing of liquid-phase water into its gaseous phase, requires a latent heat (heat of vaporization) of 597 cal/g. ’éó Evaporation is a rather complex process. Under standard atmospheric pressure, water boils at 100┬░C. At higher elevations, where the atmospheric pressure is less, water boils at temperatures lower than 100┬░C, This phenomenon may be explained best from a molecular-exchange viewpoint. ’éó At the gasŌĆöliquid interface, there is continual interchange of molecules leaving the liquid to the gas and molecules entering the liquid from the gas. 7
- 8. ’éó Net evaporation occurs when more molecules are leaving than are entering the liquid; net condensation occurs when more molecules are entering than are leaving the liquid. Equilibrium exists when the molecular exchange at the gas-liquid interface are equal over a time interval. ’éó Vapor molecules in the air exert a partial pressure on any contact surface that is known as the vapor pressure. This partial pressure combined with the partial pressures created by other gases in the atmosphere makes up the total atmospheric pressure. 8
- 9. ’éó If the temperature of a liquid is increased, the molecular energy is raised, causing a large number of molecules to leave the liquid. This, in turn, increases the vapor pressure. ’éó When the temperature reaches a point at which the vapor pressure is equal to the ambient atmospheric pressure, evaporation increases significantly, and boiling of the liquid takes place. The temperature at which a liquid boils is commonly known as the liquidŌĆÖs boiling point. For water at sea level, the boiling point is 100┬░C. 9
- 10. ’éó In a closed system (e.g., pipelines or pumps), water vaporizes rapidly in regions where the pressure drops below the vapor pressure. This phenomenon is known as cavitation. The vapor bubbles formed in cavitation usually collapse in a violent manner when they move into higher pressure regions. This may cause considerable damage to a system. ’éó Cavitation in a closed hydraulic system can be avoided by maintaining the pressure above the vapor pressure everywhere in the system. 10
- 11. 1.3 MASS (DENSITY) AND WEIGHT (SPECIFIC WEIGHT) ’éó In the International System of Units (SI),* the unit of measurement for mass is either gram or kilogram (kg). ’éó The density of a substance is defined as the mass per unit volume. ’éó It is a property inherent in the molecular structure of the substance. This means that density depends not only on the size and weight of the molecules but also on the mechanisms by which the molecules are bonded together. The latter usually varies as a function of temperature and pressure. 11
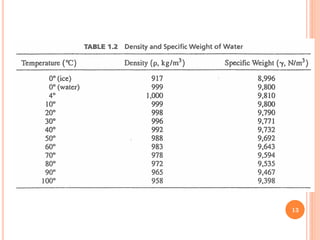
- 12. ’éó Because of its peculiar molecular structure, water is one of the few substances that expands when it freezes. ’éó The expansion of freezing water when contained in a closed container causes stresses on the container walls. These stresses are responsible for the bursting of frozen water pipes, the creation of cracks and holes in road pavement, and the weathering of rocks in nature. ’éó Water reaches a maximum density at 4┬░C. It becomes less dense when further chilled or heated. ’éó Seawater (or ocean water) contains dissolved salt. The molecules that make up the salt have more mass than the molecules they displace. Therefore, the density of seawater is about 4 percent more than that of freshwater. 12
- 13. 13
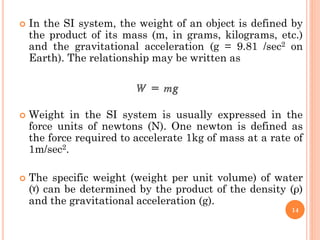
- 14. ’éó In the SI system, the weight of an object is defined by the product of its mass (m, in grams, kilograms, etc.) and the gravitational acceleration (g = 9.81 /sec2 on Earth). The relationship may be written as ’éó Weight in the SI system is usually expressed in the force units of newtons (N). One newton is defined as the force required to accelerate 1kg of mass at a rate of 1m/sec2. ’éó The specific weight (weight per unit volume) of water (╦Ā) can be determined by the product of the density (Žü) and the gravitational acceleration (g). 14
- 15. ’éó The ratio of the specific weight of any liquid at a given temperature to that of water at 4┬░C is called the specific gravity of that liquid. ’éó The unit of mass in the British system is the slug. One slug is defined as the mass of an object that requires 1lb of force to reach an acceleration of 1ft/sec2. ’éó In the British (Imperial) system, the mass of an object is defined by its weight (ounce or pound) and the gravitational acceleration (g = 32.2 ft/sec2 on Earth). The relationship is written as 15
- 16. 16
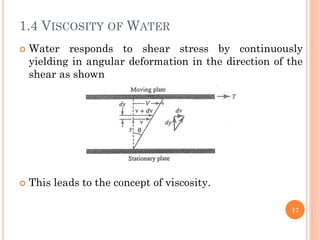
- 17. 1.4 VISCOSITY OF WATER ’éó Water responds to shear stress by continuously yielding in angular deformation in the direction of the shear as shown ’éó This leads to the concept of viscosity. 17
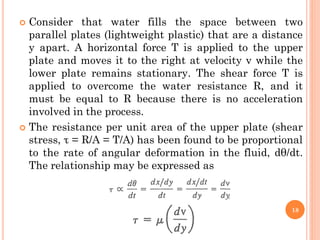
- 18. ’éó Consider that water fills the space between two parallel plates (lightweight plastic) that are a distance y apart. A horizontal force T is applied to the upper plate and moves it to the right at velocity v while the lower plate remains stationary. The shear force T is applied to overcome the water resistance R, and it must be equal to R because there is no acceleration involved in the process. ’éó The resistance per unit area of the upper plate (shear stress, Žä = R/A = T/A) has been found to be proportional to the rate of angular deformation in the fluid, d╬Ė/dt. The relationship may be expressed as 18
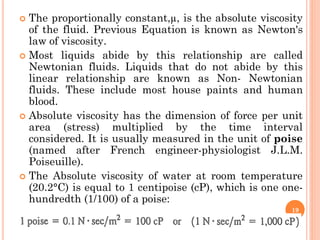
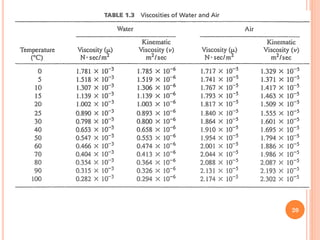
- 19. ’éó The proportionally constant,┬Ą, is the absolute viscosity of the fluid. Previous Equation is known as Newton's law of viscosity. ’éó Most liquids abide by this relationship are called Newtonian fluids. Liquids that do not abide by this linear relationship are known as Non- Newtonian fluids. These include most house paints and human blood. ’éó Absolute viscosity has the dimension of force per unit area (stress) multiplied by the time interval considered. It is usually measured in the unit of poise (named after French engineer-physiologist J.L.M. Poiseuille). ’éó The Absolute viscosity of water at room temperature (20.2┬░C) is equal to 1 centipoise (cP), which is one one- hundredth (1/100) of a poise: 19
- 20. 20
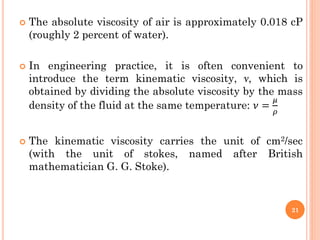
- 21. ’éó The absolute viscosity of air is approximately 0.018 cP (roughly 2 percent of water). ’éó In engineering practice, it is often convenient to introduce the term kinematic viscosity, ╬Į, which is obtained by dividing the absolute viscosity by the mass density of the fluid at the same temperature: Ø£ł = Ø£ć Ø£ī ’éó The kinematic viscosity carries the unit of cm2/sec (with the unit of stokes, named after British mathematician G. G. Stoke). 21
- 22. 22