Chapter 3 movement of substances 2011
- 1. Chapter 3- Movement of substances Chapter 3- Movement of substances
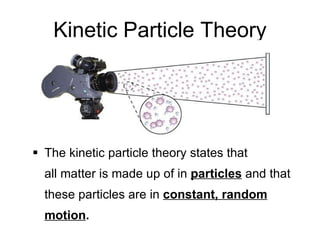
- 2. Kinetic Particle Theory The kinetic particle theory states that all matter is made up of in particles and that these particles are in constant, random motion .
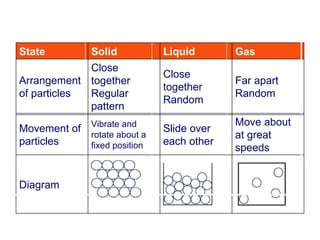
- 3. Ìı ÌıÌıÌıÌıÌıÌıÌıÌıÌı Ìı ÌıÌıÌıÌıÌıÌıÌıÌıÌı Ìı ÌıÌıÌıÌıÌıÌıÌıÌı Diagram Move about at great speeds ºİºİߣ over each other Vibrate and rotate about a fixed position Movement of particles Far apart Random Close together Random Close together Regular pattern Arrangement of p artic les Gas Liquid Solid State
- 4. very weak strong very strong Attractive forces between particles high low very low Kinetic energy of particles Gas Liquid Solid State
- 5. http://www.phschool.com/science/biology_place/labbench/lab1/intro.html Explaining of key concepts with quiz and experiments to try. http://www.nottingham.ac.uk/nursing/sonet/rlos/science/ osmosis /index.html Animations with narration Some websites to explore⦠â¦
- 6. Diffusion-Examples Getting a whiff of KFC before you step into the restaurant Watching purple ribena slowly âcolouringâ the glass of water
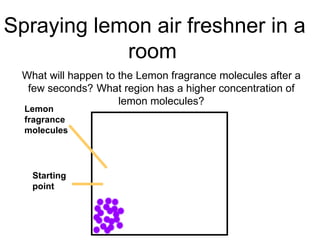
- 7. What will happen to the Lemon fragrance molecules after a few seconds? What region has a higher concentration of lemon molecules? Lemon fragrance molecules Starting point Spraying lemon air freshner in a room
- 8. Describing what you have seen⦠The 20 lemon fragrance molecules spreads out from the starting point of spraying until they are evenly spread in the room. There is a higher concentration of lemon fragrance molecules from the point of spraying compared to the surroundings of a room. This creates a concentration gradient between the two regions
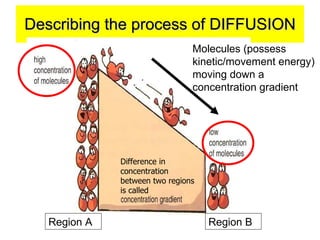
- 9. Describing the process of DIFFUSION Difference in concentration between two regions is called Region A Region B Molecules (possess kinetic/movement energy) moving down a concentration gradient
- 10. Describing the process of DIFFUSION⦠The 20 lemon fragrance molecules (possess kinetic energy as they are in random motion) diffuse from a region of higher concentration to a region of lower concentration, that is, down a concentration gradient until an equilibrium is reached.
- 11. When the lemon fragrance molecules are evenly spread, there is no net change in the system. This is known as dynamic equilibrium But does this mean that the lemon fragrance molecules have stopped movingâ¦. ? Describing the process of DIFFUSIONâ¦
- 12. NO The gas particles are still moving about as they are always in constant and random motion. [ Kinetic Particle Theory]
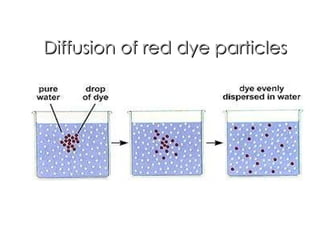
- 13. Diffusion of red dye particles
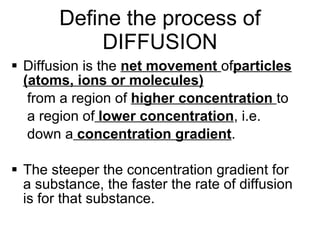
- 14. Define the process of DIFFUSION Diffusion is the net movement of particles (atoms, ions or molecules) from a region of higher concentration to a region of lower concentration , i.e. down a concentration gradient . The steeper the concentration gradient for a substance, the faster the rate of diffusion is for that substance.
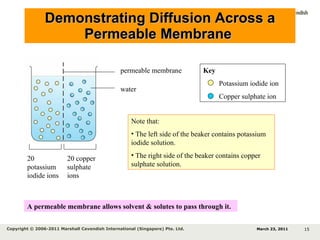
- 15. Demonstrating Diffusion Across a Permeable Membrane 20 potassium iodide ions 20 copper sulphate ions permeable membrane water Note that: The left side of the beaker contains potassium iodide solution. The right side of the beaker contains copper sulphate solution. Key Potassium iodide ion Copper sulphate ion March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. A permeable membrane allows solvent & solutes to pass through it.
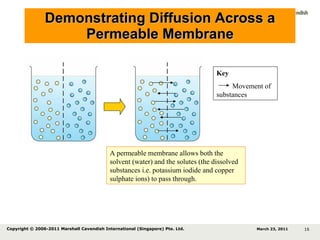
- 16. Demonstrating Diffusion Across a Permeable Membrane A permeable membrane allows both the solvent (water) and the solutes (the dissolved substances i.e. potassium iodide and copper sulphate ions) to pass through. Key Movement of substances March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
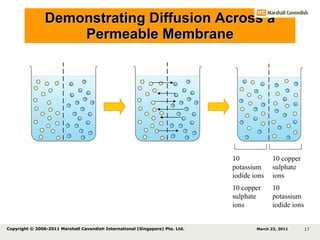
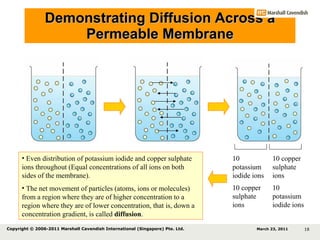
- 17. Demonstrating Diffusion Across a Permeable Membrane 10 potassium iodide ions 10 copper sulphate ions 10 potassium iodide ions 10 copper sulphate ions March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
- 18. Demonstrating Diffusion Across a Permeable Membrane 10 potassium iodide ions 10 copper sulphate ions 10 potassium iodide ions 10 copper sulphate ions Even distribution of potassium iodide and copper sulphate ions throughout (Equal concentrations of all ions on both sides of the membrane). The net movement of particles (atoms, ions or molecules) from a region where they are of higher concentration to a region where they are of lower concentration, that is, down a concentration gradient, is called diffusion . March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
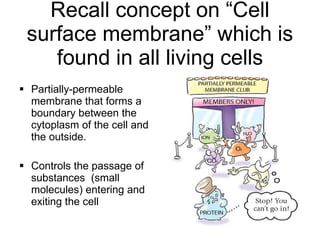
- 19. Recall concept on âCell surface membraneâ which is found in all living cells Partially-permeable membrane that forms a boundary between the cytoplasm of the cell and the outside. Controls the passage of substances (small molecules) entering and exiting the cell
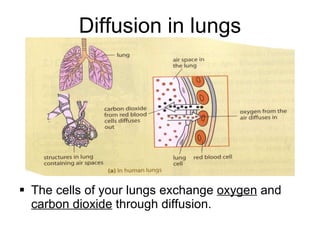
- 20. Diffusion in lungs The cells of your lungs exchange oxygen and carbon dioxide through diffusion.
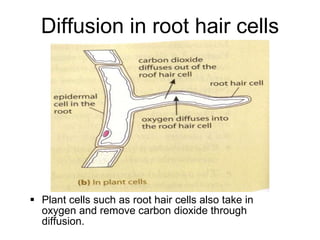
- 21. Diffusion in root hair cells Plant cells such as root hair cells also take in oxygen and remove carbon dioxide through diffusion.
- 22. How can the rate of diffusion be affected? 1. Temperature Increase in temp. increases rate 2. Size of particles Small sizes diffuse faster than large ones 3. Thickness of the barrier Thicker walls, slower rate 4. Concentration gradient Greater concentration gradient, faster rate 5. Surface area Increase S.A., increase rate
- 23. Define osmosis Osmosis is the movement of water molecules from a solution of higher water potential to a solution of lower water potential across a partially permeable membrane.
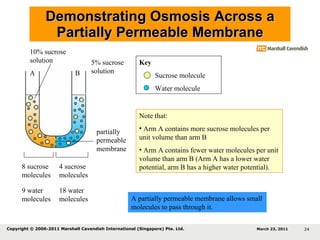
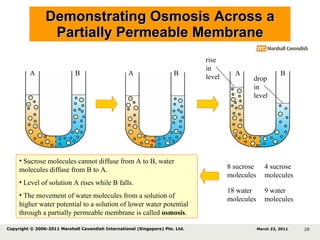
- 24. Demonstrating Osmosis Across a Partially Permeable Membrane 8 sucrose molecules 4 sucrose molecules 9 water molecules 18 water molecules Key Sucrose molecule Water molecule 10% sucrose solution 5% sucrose solution Note that: Arm A contains more sucrose molecules per unit volume than arm B Arm A contains fewer water molecules per unit volume than arm B (Arm A has a lower water potential, arm B has a higher water potential). A B partially permeable membrane March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. A partially permeable membrane allows small molecules to pass through it.
- 25. Water Potential Water potential is a measure of the tendency of water to move from one place to another. The more diluted a solution, the higher the water potential. Water always move from a solution with higher water potential to a solution with a lower one, i.e. down a water potential gradient.
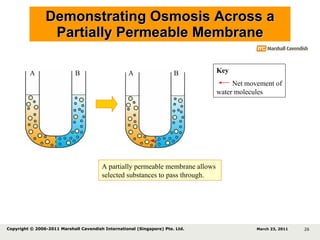
- 26. Demonstrating Osmosis Across a Partially Permeable Membrane A partially permeable membrane allows selected substances to pass through. Key Net movement of water molecules A B A B March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
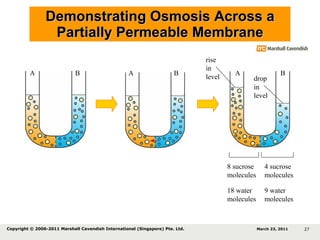
- 27. Demonstrating Osmosis Across a Partially Permeable Membrane 8 sucrose molecules 4 sucrose molecules 18 water molecules 9 water molecules A B A B A B drop in level rise in level March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
- 28. Demonstrating Osmosis Across a Partially Permeable Membrane 8 sucrose molecules 4 sucrose molecules 18 water molecules 9 water molecules Sucrose molecules cannot diffuse from A to B, water molecules diffuse from B to A. Level of solution A rises while B falls. The movement of water molecules from a solution of higher water potential to a solution of lower water potential through a partially permeable membrane is called osmosis . A B A B A B drop in level rise in level March 23, 2011 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.
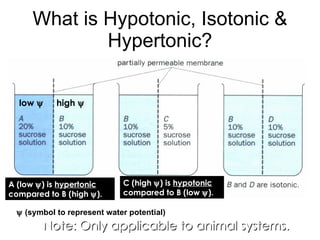
- 29. Note: Only applicable to animal systems. What is Hypotonic, Isotonic & Hypertonic? A (low ï¹ ) is hypertonic compared to B (high ï¹ ). C (high ï¹ ) is hypotonic compared to B (low ï¹ ). low ï¹ high ï¹ ï¹ (symbol to represent water potential)
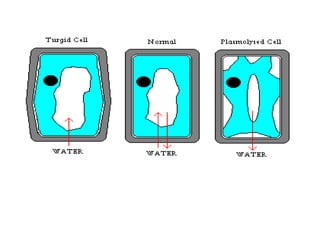
- 30. Plant cell Equal water potential inside and outside cell. No net movement of water molecules Higher water potential inside cell. Water molecules move out of cell. Cytoplasm shrinks. Cell becomes plasmolysed Higher water potential outside cell. Water molecules enter cell and exerts pressure on the cell wall. Cell becomes turgid.
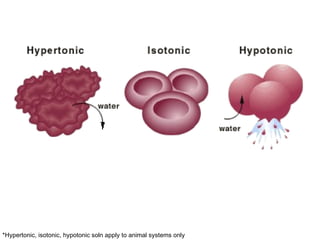
- 31. Animal cell Isotonic soln: Equal water potential inside and outside cell. No net movement of water molecules Hypertonic soln: Higher water potential inside cell. Water molecules move out of cell. Cell becomes crenated. Hypotonic soln: Higher water potential outside cell. Water molecules enter cell. Cell bursts ! *Hypertonic, isotonic, hypotonic soln apply to animal systems only
- 32. Buzz Activity What happens when living cells require certain substances that are of higher concentration inside the cell than they are in the external environment?
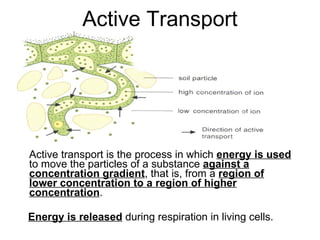
- 33. Active Transport Active transport is the process in which energy is used to move the particles of a substance against a concentration gradient , that is, from a region of lower concentration to a region of higher concentration . Energy is released during respiration in living cells.
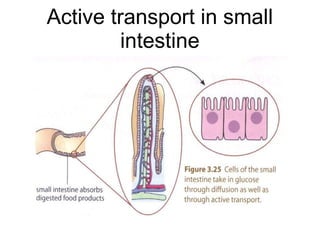
- 34. Active transport in small intestine
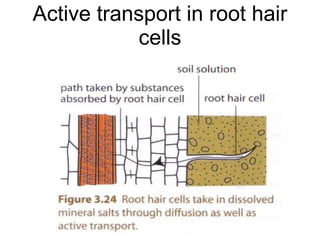
- 35. Active transport in root hair cells
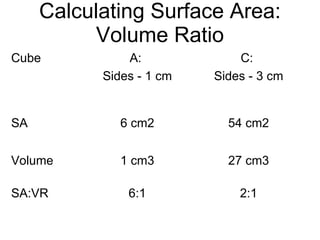
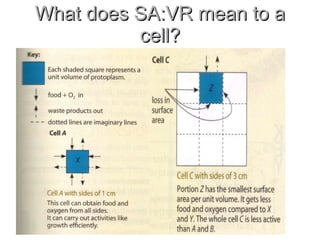
- 36. Calculating Surface Area: Volume Ratio 2:1 6:1 SA:VR 27 cm3 1 cm3 Volume 54 cm2 6 cm2 SA C: Sides - 3 cm A: Sides - 1 cm Cube
- 37. What does SA:VR mean to a cell?








![NO The gas particles are still moving about as they are always in constant and random motion. [ Kinetic Particle Theory]](https://image.slidesharecdn.com/chapter3movementofsubstances2011-110323085801-phpapp02/85/Chapter-3-movement-of-substances-2011-12-320.jpg)